Dallin Greene, DPM, reviews his surgical treatment of 1st MTP on a 51-Year-Old Female, utilizing the strong and bio-integrative OSSIOfiber® Trimmable Fixation Nail System.
Case Presentation:
A 51-year-old, 185-pound female presented to the clinic with left foot pain, secondary to a bunion deformity. She experienced pain for several years and tried inserts, padding and alternative shoe gear. Despite lifestyle changes, she had persistent pain which worsened during activity. Her profession working in a hospital required her to be on her feet. The patient had a history of smoking and continued to smoke daily. The initial assessment was moderate to severe bunion deformity to the left foot, with a large Tailor’s bunion present on her 5th metatarsal.
Surgical correction was chosen with 1st metatarsal phalangeal joint fusion and a 5th metatarsal closing wedge osteotomy on the left foot. The OSSIOfiber® Trimmable Nail, size 4.0x50mm, was utilized as adjunct fixation in conjunction with a 1st MTPJ fusion plating system.
Why was OSSIOfiber® the best choice for this patient?
With the patient’s history of smoking, I wanted to use a strong and bio-integrative OSSIOfiber Trimmable Fixation Nail in place of permanent metal hardware crossing the fusion site to enable bone growth and incorporate the implant into the surrounding bone.
Pre-Op Planning
Protocol included the patient bearing weight as tolerated in a post-op shoe with a taped bunion dressing. The wooden post-op shoe is used for 8 weeks and dressing is changed every 2 weeks. There were no deviances from my usual post-op protocol.
Surgical Technique with the OSSIOfiber® Trimmable Fixation Nail System
I followed my standard and preferred surgical procedure involving a 1st metatarsal phalangeal joint fusion with a 5th metatarsal closing wedge osteotomy and plating system to enable immediate weight bearing. I have replaced an interfragmentary screw for the OSSIOfiber Trimmable Fixation Nail as the adjunct fixation for these procedures.
The 4.0mm OSSIOfiber Trimmable Fixation Nails are provided 50mm in length. I used the provided disposable, sterile 1.1mm k-wire and cannulated drill bit to create the pilot hole. After utilizing the provided depth measurement tool, a sagittal saw was used to trim the Nail to the desired 30mm length.
The pre-trimmed Nail was loaded into the insertion sleeve and advanced into the tunnel with the tapered end first by tapping the plunger with a mallet; easily and confidently assisting in the implantation. I utilized a distal to proximal trajectory across the 1st metatarsal phalangeal joint to ensure the Nail engages cortical bone at each end.
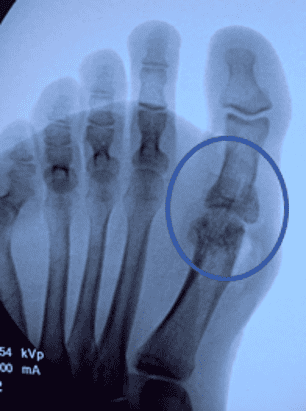
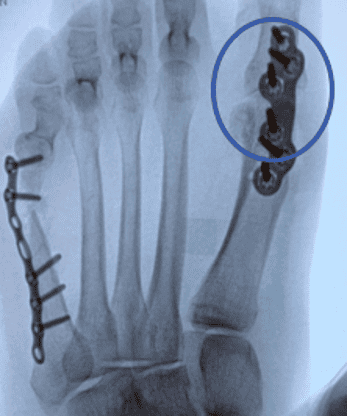
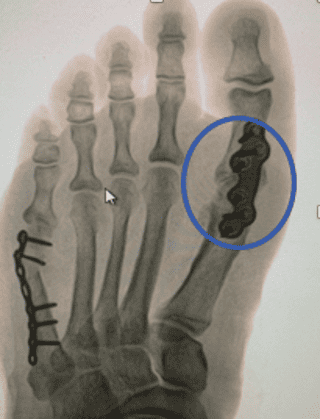
Note: OSSIOfiber material technology has a similar radiodensity to cortical bone and is artifact-free on X-ray/CT and MRI safe.
Post-Op Protocol
For this patient, I prescribed the following post-operative protocol:
- Non-weight bearing for 2 weeks
- Full weight bearing in a boot from 2-4 weeks
- Comfortable shoe if swelling allows at week 4
This slightly differs from my standard protocol to address the 5th metatarsal osteotomy by including an initial non-weight bearing stage. In comparison, for isolated 1st metatarsal severe bunion corrections, I instruct patients to partial-weight bear for 2 weeks in an Orthowedge shoe, transition to a post-operative shoe at 2 weeks, and progress to a good, supportive shoe at 4 weeks.
Summary
The 4.0x50mm OSSIOfiber Trimmable Fixation Nail was very quick and easy to use. I was genuinely surprised to experience the stability it offered. I will use it as adjunct fixation for Lapidus procedures and as primary fixation for midfoot fusions. There are many promising applications for the OSSIOfiber Technology.
Refer to the product instructions for use of warnings, precautions, indications, contraindications, and complete techniques. Medical professionals must use their professional judgement in making any final determinations for product usage and technique.
This case study was created in collaboration with Dallin Greene, DPM, and was developed from the expertise, training, and professional opinion in addition to his knowledge of the OSSIOfiber® Trimmable Fixation Nails. Results from case studies are not predictive of results in other cases. Results may vary.
Not available for sale outside the US.
DOC0001394 Rev 1.0 10/2020
