|
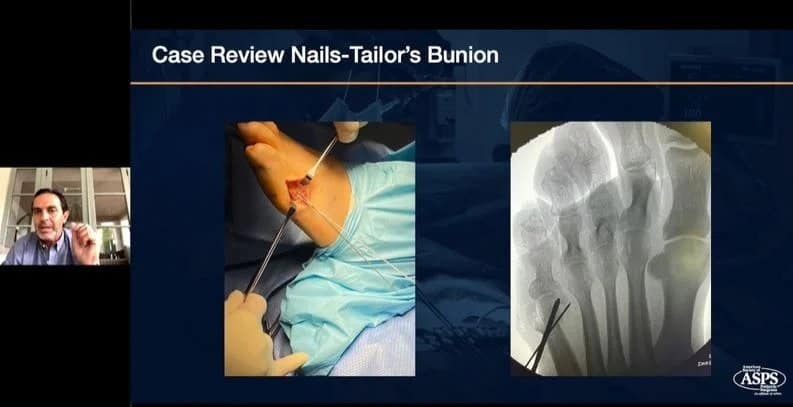
Earn CECH credits by watching this recent webinar, hosted with American Society of Podiatric Surgeons and presenter Bob Baravarian, DPM, FACFAS. NOW ON-DEMANDWEBINAR: The Science and Clinical Experience with Strong and Bio-Integrative Implant Fixation Speaker: Bob Baravarian, DPM, FACFAS
Hear Dr. Baravarian present on:
Log in to the ASPS website to access the webinar and earn CECH credit! Please note to receive CECH credit, active ASPS membership or additional access may be required. |
ASPS Webinar
Related Posts
All-Natural OSSIOfiber® Flatfoot Reconstruction
Author: Dallin Greene, DPM, Glendive Medical Center in Glendive, MT. Dr. Dallin Greene is a board-certified Foot and Ankle surgeon, treating all aspects of the foot and ankle with expertise in deformity correction. Dr. Greene graduated from Brigham Young University-Idaho in 2009 with a bachelor’s in biology. He then attended Midwestern University Arizona for aContinue reading “All-Natural OSSIOfiber® Flatfoot Reconstruction”
Midfoot Revision Arthrodesis Using OSSIOfiber® Nail & Screws Following Naviculocuneiform Nonunion
Author: Cody J. Togher, DPM at The Joint Replacement Institute. Dr. Togher is a Fellowship-Trained Foot & Ankle Surgeon at the Joint Replacement Institute in Naples, FL. He completed his residency at AdventHealth East Orlando prior to completing fellowship at the Orthopedic Foot & Ankle Center in Columbus, OH. Dr. Togher specializes in complex footContinue reading “Midfoot Revision Arthrodesis Using OSSIOfiber® Nail & Screws Following Naviculocuneiform Nonunion”
Bilateral Hallux Valgus Correction with All-Natural, Bio-Integrative Lapidus Procedure
Surgeon: Mark A Prissel, DPM FACFAS, Board-Certified, Fellowship Trained Foot & Ankle Surgeon Education/training: Dr. Prissel is a fellowship trained foot and ankle surgeon and partner at The Orthopedic Foot and Ankle Center (OFAC), a practice devoted to multi-disciplinary foot and ankle care in Worthington, Ohio. In addition to his surgical practice, Dr. Prissel currentlyContinue reading “Bilateral Hallux Valgus Correction with All-Natural, Bio-Integrative Lapidus Procedure”