Author: Kevin Palmer, DPM at Golden Orthopedics, Boca Raton Outpatient Surgery and Laser Center. Practice includes trauma, reconstruction, elective forefoot/rearfoot procedures, sports medicine and wound care.
Dr. Palmer completed his residency at Westside Regional, Plantation, FL. Board certified by the American Board of Podiatric Surgery, in Foot Surgery, Rearfoot Reconstructive Ankle Surgery.
INTRODUCTION
Ankle fractures are one of the common presentations in the orthopedics speciality, with approximately 20% of patients presenting concomitant syndesmotic injury. Trans-syndesmotic screw fixation is a highly effective method for achieving stable fixation, usually with one or more syndesmotic screws. Interestingly, patients with broken metal screws reported better clinical outcomes than those with retained intact metal screws, likely due to preserved physiological tibiofibular movement. Removal of syndesmotic screws remains common practice yet requires a second procedure which is cumbersome to both patients and health system and is not without complications.1-3
CASE PRESENTATION
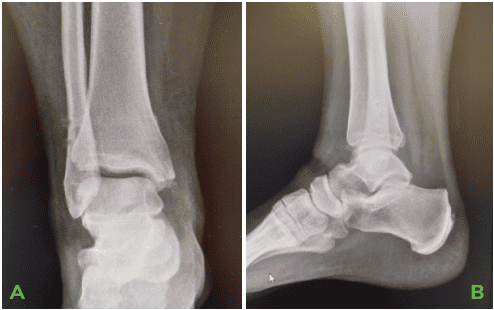
A 34-year-old, Female patient with BMI of 43.57, presented with a painful left ankle unable to bear weight after suffering a fall. X-rays demonstrated transverse medial malleolus fracture, syndesmosis rupture and a small posterior malleolar fracture consistent with trimalleolar fracture pattern.
WHY OSSIOfiber® IS AN IDEAL CHOICE FOR THIS PATIENT?
This is a young patient who would likely require removal of hardware in the future due to her syndesmotic injury. The decision was made in favor of single procedure using OSSIOfiber® Compression Screws, as they provide the desired stable fixation, with no need for hardware removal.
Preoperative Planning:
ORIF of a displaced transverse medial malleolar fracture and syndesmotic widening. A small non-displaced posterior malleolar fracture was assessed as too small for internal fixation.

Surgical Procedure:
ORIF medial malleolar fracture and syndesmosis rupture right ankle.
- Medial Malleolus fracture repair using 2X OSSIOfiber® 4.0 CS x 50mm
- Syndesmosis injury repair using 2X OSSIOfiber® 4.0 CS (46mm & 50mm)
Surgical Technique Steps:
ORIF medial malleolar fracture and syndesmosis rupture right ankle.
- Surgical approach:
- Incision over medial malleolus, medial periosteal incision of approximately 4.5 cm, to visualize fracture.
- Lateral incision over distal fibula at site of planned screw insertion.
- Linear periosteal incision over distal fibula.
- Fixation site preparation:
The medial malleolus fracture site was debrided, irrigated, and reduced using a standard pointed reduction clamp. Intra operative fluoroscopy was used to confirm proper alignment. A large reduction clamp was used to stabilize the lateral fibula to anterior medial tibia to reduce the syndesmosis to anatomic alignment. - Tunnel preparation:
Medial Malleolus: Guide wires were inserted in standard fashion across medial malleolus fracture and desired placement under fluoroscopy was confirmed. A controlled amount of countersink was performed medially and appropriate screw length measured. Tapping for the screw threads was performed per the surgical technique guide.
Syndesmosis: Guide wires inserted in parallel fashion from fibula into medial tibia. Note: For Syndesmotic screws, more countersink is needed due to density of the bone (Fibula cortex is much harder than medial malleolus). Also, the screws must be placed in the center of the fibula during insertion to prevent stress riser/fracture. Tapping of the syndesmotic screws was performed per the described technique - Implant Insertion
Both the Medial malleolar and syndesmotic screws were inserted using slow power, and hand tightened as needed. - Final imaging confirmed well-aligned fracture fixation and reduction of the Syndesmosis.
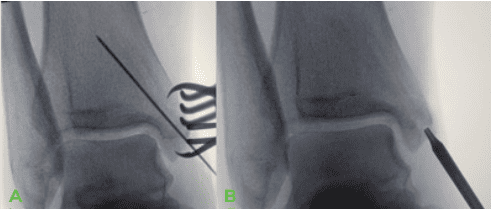
and bone reduction clamps. B. OSSIOfiber® CS insertion, screwdriver seated in screw hex.
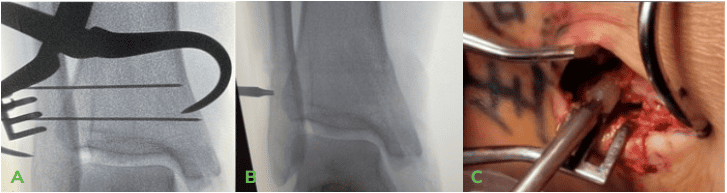
Technique Pearls:
- Consider the amount of countersinking according to bone quality. Countersinking should be kept to a minimum in the presence of poor bone quality. Conversely, full countersinking should be achieved within stronger healthy bone.
- ‘Listen’ to the screws as they are tightened – the screw provides audible feedback indicating tightness.
Post-Op Protocol:
The use of the OSSIOfiber® CS does not alter the standard post-operative instructions for these cases. Patient Non weight bearing for 6 weeks followed by partial weight bearing for 6-10 weeks in ankle brace/sneaker.
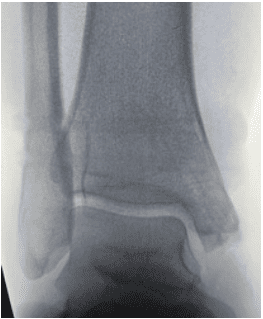
Patient Follow-Up at 25 Weeks:
Healing progresses as expected for the time interval. Patient is happy to not see hardware, as the screws are virtually undetectable on radiographic images due to OSSIOfiber® radiodensity similar to cortical bone.
Summary:
OSSIOfiber® screws are an ideal product for this indication. While the screws provide the mechanical strength needed for bone fixation during healing, we can preserve physiological mobility and avoid a second hardware removal procedure. OSSIOfiber® implants can be a great option even if used in combination with metallic implants, reducing metal hardware to a minimum. When thinking of patients who require multiple surgeries, usually as a result of traumatic or degenerative type of pathologies, this product will simplify future surgical treatment plans. OSSIOfiber® can also help maintain bone stock and avoid defects caused by conventional hardware removal. There are many possibilities as additional OSSIOfiber® bio-integrative products come to market.
References
1. Hunt KJ. “Syndesmosis injuries.” Current reviews in musculoskeletal medicine vol. 6,4 (2013): 304-12.
2. Desouky O, Elseby A, Ghalab AH. “Removal of Syndesmotic Screw After Fixation in Ankle Fractures: A Systematic Review”. Cureus. 2021;13(6):e15435.
3. Walley KC, Hofmann KJ, Velasco BT, Kwon JY. “Removal of Hardware After Syndesmotic Screw Fixation A Systematic Literature Review”. Foot and Ankle Specialist. 2016 DOI: 10.1177/1938640016685153.
Medical professionals must use their professional judgment for patient selection and appropriate technique.
Results from case studies are not predictive of results in other cases. Results may vary.
Please refer to the product instructions for use for warnings, precautions, indications, contraindications and technique.
Not available for sale outside of the US. Speak to your local sales representative for product availability.
For more information, please visit ossio.io
® OSSIO and OSSIOfiber® are registered trademarks of OSSIO Ltd.
All rights reserved 2022.
DOC-002300 Rev 1.0
