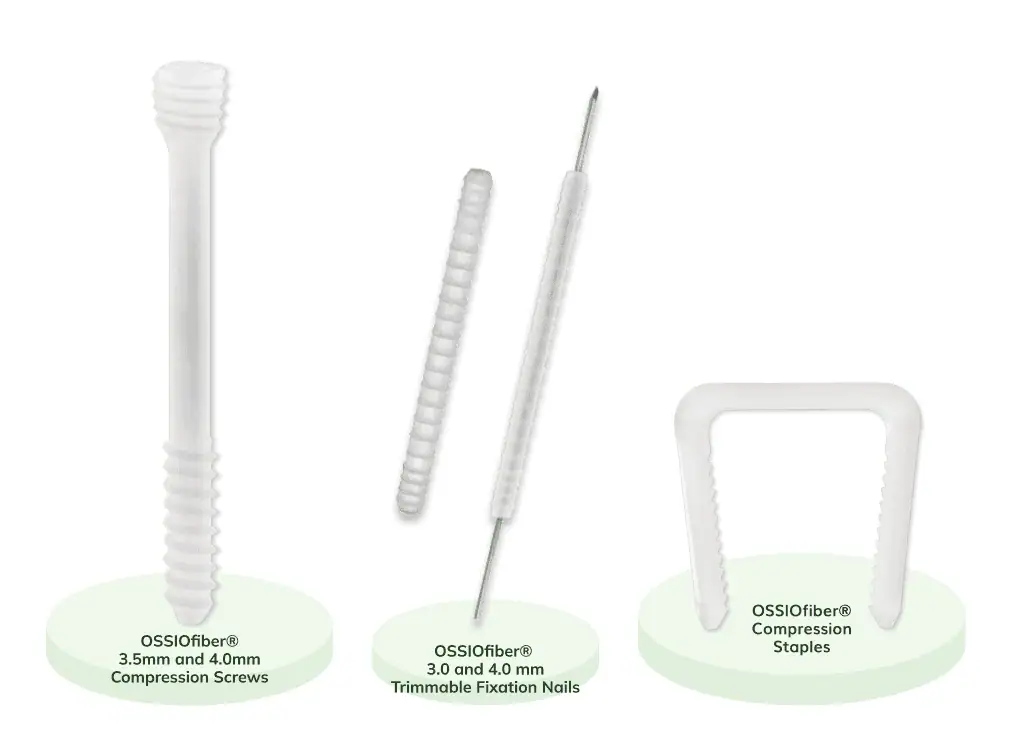
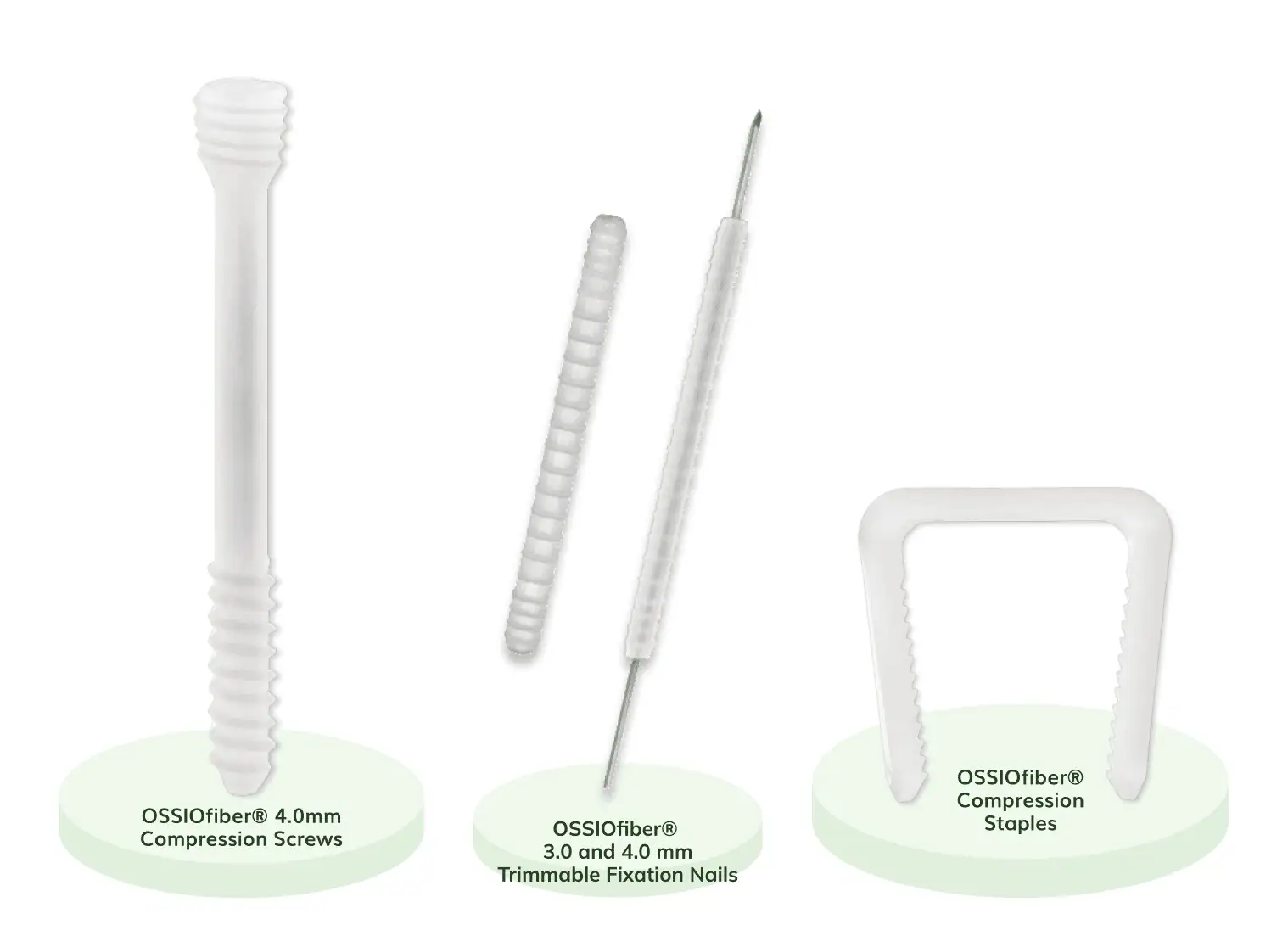
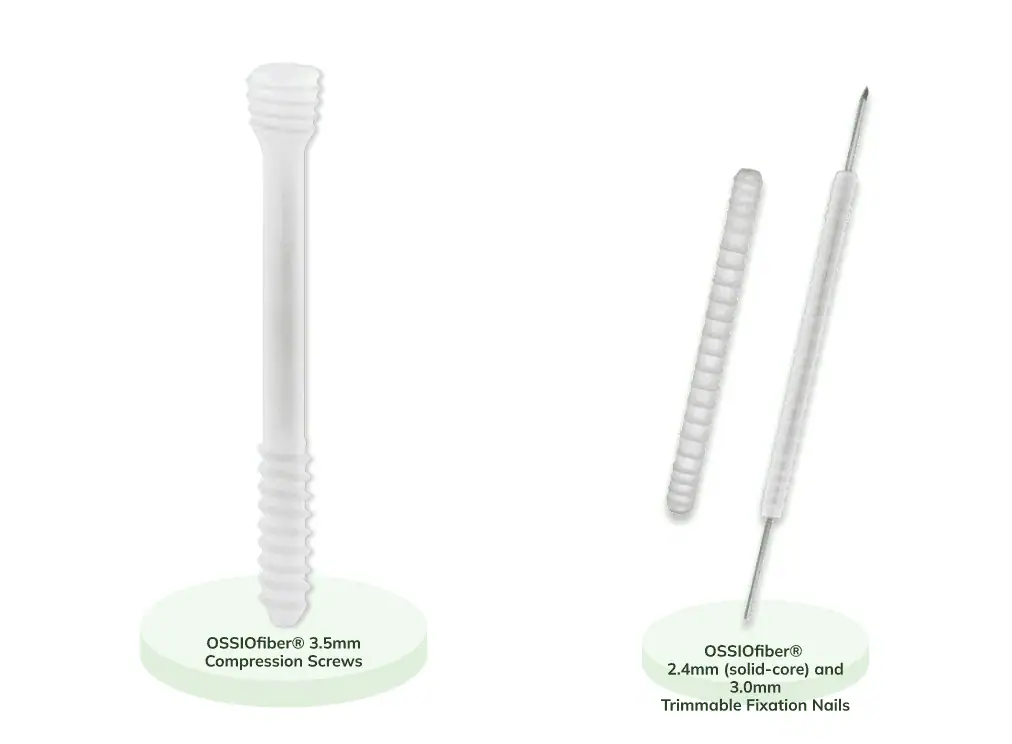
OSSIOfiber®
All Bunion Patients Are Not The Same.
Why Treat Them That Way?

Bio-Integrative OSSIOfiber® Implants:
The First Credible Alternative to Metal Hardware
Add The OSSIOfiber® Metal-Free Option For Your Bunion and Hammertoe Patients*
*Refer to IFU of each OSSIOfiber product for a full list of indications, contraindications etc.

All-Natural First Metatarsophalangeal Joint Arthrodesis with OSSIOfiber®Trimmable Nail and OSSIOfiber® Compression Staple
The use of OSSIOfiber® fixation in this patient afforded successful clinical and radiographic correction of a hallux rigidus deformity without the use of retained metallic implants. In addition to the long-term benefits associated with bio-integrative fixation…
OSSIOfiber®
Signature Qualities

1.5x Stronger than Cortical Bone

Early Bone Attachment in as little as 2 weeks

Natural Environment for Bone Regeneration

Complete Integration in 24 Months

No Adverse Inflammation