Our proprietary material technology, OSSIOfiber® Intelligent Bone Regeneration Technology, has unique material composition and structural design that contribute to its strength and optimal bio-integration for orthopedic fixation. Scientific and clinical leaders at OSSIO discuss the science behind OSSIOfiber in this Technology Overview White Paper.
Key Takeaways:
- OSSIOfiber material composition creates the ideal bone fixation material that supports mechanical strength and balanced pH environment favorable for bone regeneration
- The micro-architecture of thousands of continuous natural mineral fibers is engineered to create the optimal biomechanical properties in different mechanical axes for the clinical indications
- As OSSIOfiber implants degrade, interconnected pores are formed allowing for fluid flow and regeneration of bone tissue in place of the implant
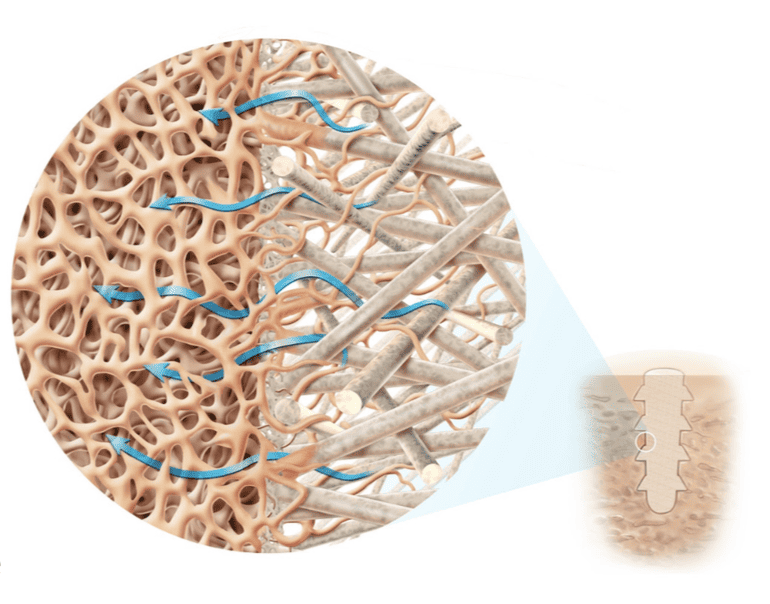
Through the material composition and internal architecture, OSSIOfiber is designed to provide both the mechanical strength required for insertion and secure fixation while having the ability to integrate into native bone without adverse inflammation. In doing so, OSSIOfiber Intelligent Bone Regeneration Technology creates a new category of fixation, called ‘Bio-Integratives’.
Learn more about material composition and structural design of OSSIOfiber Intelligent Bone Regeneration Technology.
