Author: Kevin Palmer, DPM at Golden Orthopedics, Boca Raton Outpatient Surgery and Laser Center. Practice includes trauma, reconstruction, elective forefoot/rearfoot procedures, sports medicine and wound care.
Dr. Palmer completed his residency at Westside Regional, Plantation, FL. Board certified by the American Board of Podiatric Surgery, in Foot Surgery, Rearfoot Reconstructive Ankle Surgery.
INTRODUCTION
Metatarsal fractures are the most common of all foot and ankle related fractures (88%).1 Fractures can occur anywhere on the metatarsal bone with no specific fracture classification, they can be isolated, multiple or occur in combination with fracture-dislocations of the Lisfranc joint. The goal of treatment is to obtain bone healing while maintaining the metatarsal parabola, the sagittal position of the metatarsal heads and bone-to bone contact, to preserve a functional forefoot. The choice of the operative technique depends on the fracture characteristics. Traditional repair of the 1st Tarsometatarsal (TMT) fracture-dislocation usually require a metal plate & screw fixation method, while for neck metatarsal fractures, k-wires are commonly utilized in a percutaneous fashion and removed 6-8 weeks later. Complications that can be seen with existing techniques include painful hardware and the need for hardware removal, metatarsalgia in up to 56.8% of patients, hypertrophic scars and painful calluses, metatarsophalangeal plantar plate lesions, and infectionction.1-3
CASE PRESENTATION
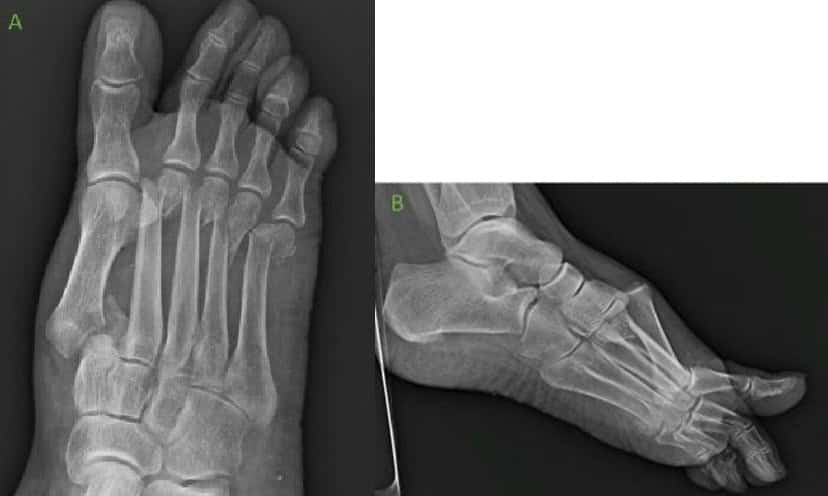
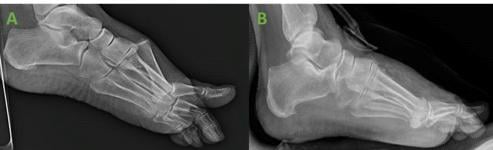
A 69-year-old, Female patient with BMI of 29, presented with a right foot injury after a fall following a syncopal episode. The foot was placed in a well-padded fiberglass splint. On examination unable to bear weight with moderate pain of right foot. X-rays reveal dorsally displaced fracture at the base of the 1st metatarsal, and displaced fractures of the 2nd-5th metatarsal necks. No signs of compartment syndrome observed. The patient’s medical history involves – Hypercholesterolemia, Hypothyroidism, and Gastroesophageal reflux disease (GERD). No known drug allergy and patient denied past surgical history (PSH).
WHY OSSIOfiber® IS AN IDEAL CHOICE FOR THIS PATIENT?
Due to the known complications of common metatarsal fracture treatment options, and taking into account the likelihood for hardware removal in this case, it was determined that OSSIOfiber® would be the ideal choice of treatment for this patient, providing stable fixation and stabilization for the healing period while avoiding the use of permanent hardware. Compression Screws (CS) could be used to repair the dislocated 1st TMT fracture in a 2-screw parallel fashion, and Trimmable Fixation Nails (TFN) will be utilized in an intramedullary fashion to repair all metatarsal fractures, leaving no prominent hardware/wires.
Preoperative Planning:
1st metatarsal base Fracture with dorsal dislocation: ORIF was decided upon, using parallel crossing OSSIOfiber® Compression Screws to reduce this fracture and stabilize the joint itself. Lesser Metatarsal neck Fractures: ORIF using OSSIOfiber® TFN. Planned incisions at 2nd and 4th interspaces (IS) to access also adjacent fractures with minimal dissection, maintaining healthy skin bridge between each incision. Incisions planned approximately 2cm in length.
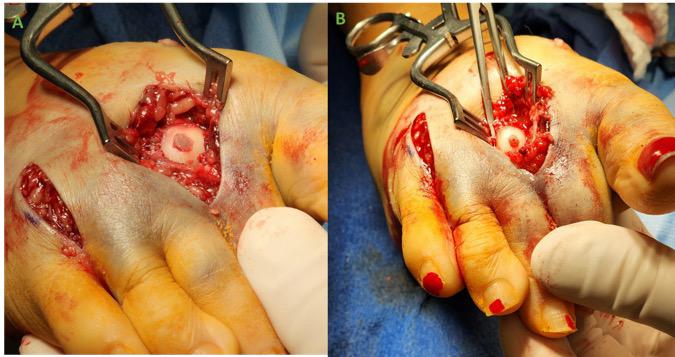
Surgical Procedure:
- The 1st TMT fracture fixation done using 2X OSSIOfiber® 4.0 CS (34mm & 50mm).
- Lesser Metatarsal neck Fractures fixation was done using OSSIOfiber® TFNs in several configurations:
- OSSIOfiber® TFN 2.4X30mm – two nails
- OSSIOfiber® Cannulated TFN 3.0X50mm
- OSSIOfiber® TFN 4.0X50mm
Surgical Technique Steps:
- Dissection / Access
Dorsomedial incision over base of 1st metatarsal base fracture/1st TMT dislocation site. Metatarsal necks interspace incisions approximately 2 cm created at 2nd IS, and 4th IS. - TMT Fixation site preparation:
Reduction and stabilization of 1st metatarsal base fracture/dislocation essential before OSSIOfiber® CS placement (0.062” k-wires used to stabilize reduction). Once anatomic reduction was achieved to the 1st metatarsal base fracture, utilizing a point-to-point bone clamp and K-wires, a dorsal/midshaft to plantar/ medial cuneiform OSSIOfiber® CS was placed using the provided cannulated wire/drill/gentle countersink techniques. A second OSSIOfiber® CS was placed in a parallel fashion superior to the first screw, into the central portion of the medial cuneiform body. - Metatarsal necks tunnel preparation:
This was prepared in a standard fashion with cannulated guide wire and respective drill for each OSSIOfiber® TFN. Guide wire was started in the central aspect of the metatarsal head and over drill performed to adequate intramedullary depth. The 2nd metatarsal fracture was fixed with 4.0mm nail, 3rd metatarsal fracture was fixed with 3.0mm nail, and 4th/5th fixed with 2.4mm nail. - Implant insertion:
OSSIOfiber® CS were inserted under power instrumentation until close to tight and then hand tightened for the last few threads. OSSIOfiber® TFN were tamped gently across the metatarsal necks, with care being taken not to lose reduction or cause malalignment. The nails were then cut flush with the cartilaginous surface of the respective metatarsal, utilizing a backhanding motion with the saw, they are contoured flush to the metatarsal head contour.
In the case of the 4th metatarsal, the oblique fracture necessitated placing a Cerclage Maxforce Suture Tape for additional reduction/stabilization.
Technique Pearl:
- TMT proximal drill must be gentle so screw head will bite well into dorsal 1st metatarsal base. Cautious insertion of the screws is performed since it is easy to over-bury the head if too much countersink performed (prefer to perform minimal countersink to 1st metatarsal insertion site).
- Closure
Standard closure with 2.0 Vicryl and 3.0 Nylon sutures.
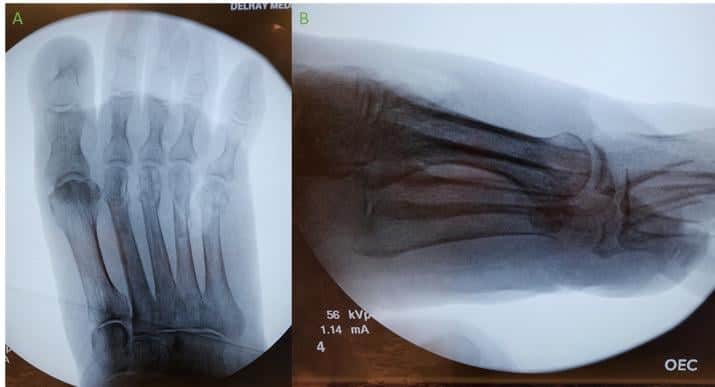
Post-Operative Protocol:
Using OSSIOfiber® bio-integrative implants enabled me to modify the post-op protocol by allowing earlier weightbearing, since there is no external protruding hardware. Also, OSSIOfiber® implants demonstrate strong hold with bone, with less concern of implant migration/loss of fixation. The common post-surgery protocol is detailed below:
- Non-weight bearing: 2-3 weeks in posterior splint
- Partial weight bearing: 4-6 weeks in CAM boot
- Full weight bearing: 7-10 weeks full WB in CAM walker
- Comfort shoes: 10-12 weeks following surgery.
Patient Follow-Up:
Follow-up visits occurred at regular intervals of – 1,2,6, 10 weeks, and 4-months post-operatively. No positional change to the bone fixation sites was noted throughout healing. Lateral X-rays at 10-weeks show well reduced 1st TMT joint with no recurrence of fracture dislocation. X-ray images taken 4 months after surgery, demonstrate maintained alignment for all fixation sites with complete healing and bone fusion.
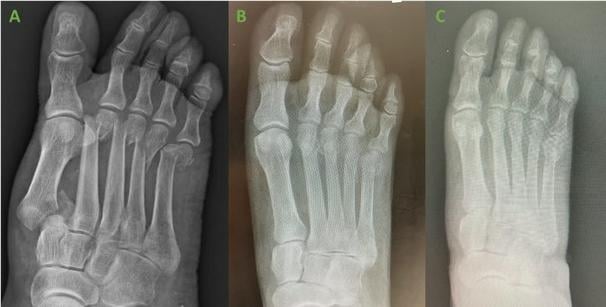
C. post-surgery.
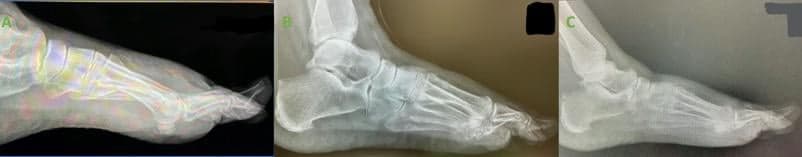
Summary:
OSSIOfiber® implants allow great fixation stability without the potential need for a difficult & painful hardware removal process. Additionally, this case presents a unique way to repair metatarsal neck fractures without the need for external, percutaneous wire fixation, which comes with its own complications and side effects. Furthermore, using OSSIOfiber® technology, which incorporates into bone, minimizes future surgical challenges in case there is a need for future procedures to the metatarsals or the 1st TMT joint.
References
1. Samaila, EM., Et al. Central Metatarsal Fractures: a Review and Current Concepts. Acta Biomed 2020; Vol. 91, Supplement 4: 36-46.
2. Baumfeld, D., Et al. Anterograde Percutaneus Treatment of Lesser Metatarsal Fractures: Technical Description and Clinical Results. Rev Bras Orthop. 2012; 47(6): 760-4 (Sociedade Brasileira de Orthopedia e Traumatologia).
3. Stravrakakis, IM., Et al. Operative Treatment of Acute Shaft and Neck Lesser Metatarsals Fractures: a Systemic Review of the Literature. European Journal of Orthopedic Surgery and Traumatology. Jan 23, 2021.
Medical professionals must use their professional judgment for patient selection and appropriate technique.
Results from case studies are not predictive of results in other cases. Results may vary.
Please refer to the product instructions for use for warnings, precautions, indications, contraindications and technique. Not available for sale outside of the US. Speak to your local sales representative for product availability.
For more information, please visit ossio.io
® OSSIO and OSSIOfiber® are registered trademarks of OSSIO Ltd. All rights reserved 2023.
DOC-002300 Rev 1.0
