Author: Erik J. Carlson, MD. Dr. Carlson is the Chief and Associate Medical Director of Orthopaedic Surgery at St. Mary’s Hospital in Waterbury, CT. In addition to his upper extremity surgical private practice, he serves as a clinical instructor for the Plancher Orthopaedic and Sports Medicine Fellowship.
INTRODUCTION
Current standard of care techniques for metacarpal fracture repairs using traditional metal implants are well documented and often lead to pain and secondary procedures. K-Wires have been associated with skin infection, osteomyelitis, and tendon injury, with rates up to 15%1. Metallic plate fixation has been associated with rates of digital stiffness of 10% and need for hardware removal in 8%2. While the intramedullary technique for metacarpal fractures has improved results, inherent issues associated with metal implants still exist. The use of OSSIOfiber® Trimmable Fixation Nails adds additional benefit by reducing the published risks associated with metallic implants in intramedullary metacarpal fixation, including hardware removal for nickel allergy, screw deformity, and screw breakage3.
CASE PRESENTATION
A 23-year-old, right-hand dominant, male presented with a tender and swollen hand sustained from a fall three days prior. His initial presentation showed a closed, minimally displaced fracture of the 5th metacarpal neck with some comminution. There was reasonable alignment and no significant rotation or angulation on initial clinical examination. A splinting and early motion protocol was initiated.
On follow-up evaluation, x-ray showed increased displacement, and a reported cross-over of the small finger onto the ring finger on digital flexion. Surgical management was discussed, and the patient elected to proceed with closed versus open reduction with internal fixation.
Pre-op Planning:
X-Rays showed a comminuted and displaced 5th metacarpal neck fracture. Intramedullary retrograde fixation with an OSSIOfiber® Trimmable Fixation Nail was recommended to provide rigid fixation and allow early motion without the potential complications associated with metallic implants.
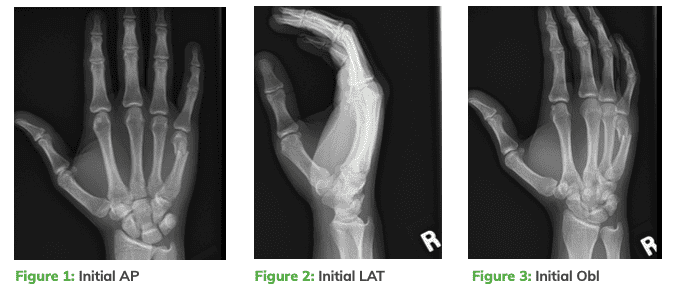
Surgical Technique
In the Operating Room, a retrograde intra-medullary approach was chosen to minimize incision, dissection, tendon irritation, and fracture exposure. The fracture was indirectly reduced and a 4.0x50mm OSSIOfiber® Trimmable Fixation Nail was used for primary fixation.
- Dissection: A longitudinal incision was made over the 5th metacarpal phalangeal joint and distal aspect of the metacarpal. The extensor tendon was split, and the articular surface of the 5th metacarpal head was visualized. Care was taken not to damage the articular surface.
- Fixation Site Preparation: The provided K-Wire was placed above the equator of the 5th metacarpal head. The fracture was reduced manually, and the K-Wire was advanced through the metacarpal intramedullary canal. Intra-operative imaging confirmed reduction and pin placement.
- Tunnel Preparation: The cannulated drill for the OSSIOfiber® 4.0mm Trimmable Fixation Nail was used to prepare the intra-medullary canal. The K-Wire was removed, and the fracture held reduced.
- Implant Insertion: The Nail was advanced across the fracture site and buried beneath the subchondral bone of the 5th metacarpal head. Fluoroscopic and visual examination demonstrated reduction of the fracture and stability with passive motion.
Post-Op Planning and Follow-Up Outcomes
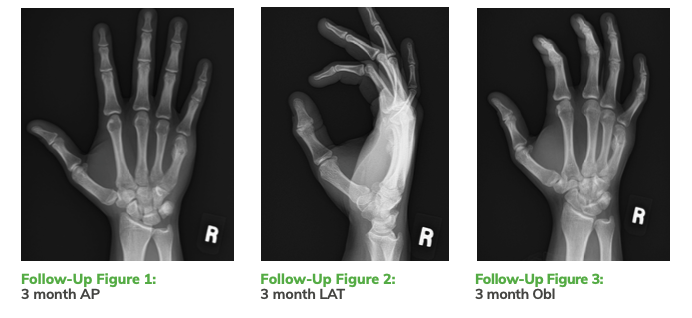
Post-Op Planning and Follow-Up Outcomes (cont’d)

Summary:
The OSSIOfiber® Trimmable Fixation Nail allowed for early stable fixation in this patient without the potential complications of metallic implants. The fracture healed routinely and there were no reported or observed adverse events or complications. Future use of the OSSIOfiber® cannulated nails and compression screws will provide for broader application within hand and upper extremity surgery while avoiding the pitfalls of the traditionalmetallic implants.
References
1. Stahl S, Schwartz O. Complications of K-wire fixation of fractures and dislocations in the hand and wrist. Arch Orthop Trauma Surg 2001;121:527–530.
2. Fusetti C, Della Santa DR. Influence of fracture pattern on consolidation after metacarpal plate fixation. Chir Main 2004;23:32–36.
3. Warrender WJ, Ruchelsman DE, Livesey MG, Mudgal CS, Rivlin M. Low Rate of Complications Following Intramedullary Headless Compression Screw Fixation of Metacarpal Fractures. Hand (N.Y.) 2020; 15(6): 798–804.
DOC-0001972 Rev 02 1/2022
For more on OSSIO and OSSIOfiber,
please visit ossio.io or call 833-781-7373
® OSSIO and OSSIOfiber are registered trademarks of OSSIO Ltd. All rights reserved. Not available for sale outside of US. Speak to your local sales representative for product availability.
Refer to the product Instructions for Use for warnings, precautions, indications, contraindications, and technique.
OSSIO Inc. 300 Tradecenter Drive, Suite 3690, Woburn, MA 01801
