Author: Dr. Kelsey Millonig, DPM, MPH, FACFAS, East Village Foot & Ankle Surgeons Clinic, IA.
Dr. Millonig is a double board certified fellowship trained foot and ankle surgeon with East Village Foot and Ankle Surgeons in Des Moines, IA. She completed her medical education at Des Moines University earning dual degrees in Doctor of Podiatric Medicine and Masters in Public Health. She was the first podiatrist to be selected as an intern at the World Health Organization Headquarters in Geneva, Switzerland. Dr. Millonig completed her surgical residency at Franciscan Foot and Ankle Institute. She then completed her complex deformity correction and orthoplastic reconstructive fellowship at the Rubin Institute of Advanced Orthopedics International Center for Limb Lengthening in Baltimore, MD. Dr. Millonig has served as a medical missionary in several countries. She has authored multiple articles in medical journals and textbooks. Dr. Millonig frequently lectures nationally, has earned several scholarly awards, and held multiple leadership positions in numerous national podiatric and public health committees.
INTRODUCTION
The talus is the second largest bone of the foot, has a fundamental role in ankle stability and normal foot and ankle movement1,2. It has no muscular attachments, and over one half of its surface is covered by articular cartilage. The talus is supported solely by the surrounding joint capsules, ligaments, and synovial tissues2,3. It is a weight bearing bone, and when injured, the collective motion of the foot and ankle becomes compromised, and significant disability or chronic pain can result4. Studies have shown that each 1mm of talar shift results in roughly 40% decrease in the contact surface area at the tibiotalar joint. Such a change can precipitate the development of arthritis, a degenerative disease that is painful and debilitating5. Osteochondral lesions of the talus are a relatively frequent pathology that foot and ankle surgeons encounter, caused by a single or multiple traumatic events6,7. Lesions of the talar shoulder, as in this documented case, present an even more difficult challenge. Lesions that are not surrounded by articular cartilage on the shoulder are uncontained lesions which have been shown to have lower success rates of treatment regardless of lesions size or location7. Traditionally, the mainstay of surgical treatment consists of arthroscopic inspection, debridement of surrounding soft tissue pathology, and microfracture bone marrow stimulation. In failed attempts at subchondral bone healing, total ankle replacement (TAR) or ankle arthrodesis is considered as a means to reduce pain and restore joint stability and mobility5. Allograft procedures are a viable treatment of talar surgeries due to limited treatment options available. Cartilage viability is one of the most important factors for successful clinical outcomes after transplantation of osteochondral allografts and is related to storage length and intraoperative factors8. Bio-integrative OSSIOfiber® Trimmable Fixation Nails (TFN) are fixation devices made of PLDLA reinforced with continuous mineral fibers. The nails are indicated for maintenance of alignment and fixation of bone fractures, osteotomies, arthrodesis and bone grafts. They are designed to provide the desired stability, while integrated with the surrounding bone over time. Allograft fixation using these bio-integrative nails supports the biological procedure, leaving behind a viable environment rather than any permanent hardware.
CASE PRESENTATION
A 67-year-old female patient, 170lbs, non-diabetic, non-smoker with medical history of Hypothyroidism and Osteoporosis. The patient first started experiencing left ankle pain 3 years prior to her current presentation, with no inciting acute trauma. She was diagnosed with subchondral insufficiency fracture with osteochondral lesion of the talar dome and underwent two previous surgeries from her former surgeon.
The first surgery included arthroscopic debridement of the left ankle; subchondral drilling and injection of calcium phosphate bone graft substitute into the medial malleolus and medial talar dome cystic lesions as well as an Anterior Talo-Fibular ligament (ATFL) Brostrom-Gould repair. As pain persisted a second ankle surgery took place 7-months later, consisting of tibial core decompression of a solitary distal tibial cyst utilizing a cortical window resection of the distal tibia; curettage and irrigation of the cystic lesion; and packing of bone substitute with replacement of the cortical window. The talar cartilage was noted to be intact intraoperatively at this time. The patient continued to have pain which worsened over time. An MRI taken 1-year post second surgery demonstrated progression of subchondral edema at the medial half of the talar dome as well as subchondral cystic changes to this location. An 11 x 9mm Osteochondral Defect (OCD) fragment of the medial talar shoulder was identified.
Upon her initial visit to the clinic following the above treatment, the patient complained of pain to her left ankle. On physical exam: Patient had a painful ankle range of motion, pain on palpation over left lateral fibular exostosis, left posterior tibial tendon, and left deltoid ligament. Most notably, she had substantial pain on palpation of medial gutter of the ankle joint. She had a negative talar tilt test and with no pain on manipulation of the syndesmosis. Biomechanically she had a neutral Resting Calcaneal Stance Position (RCSP) and mild overpronation.
PRE-OPERATIVE IMAGING
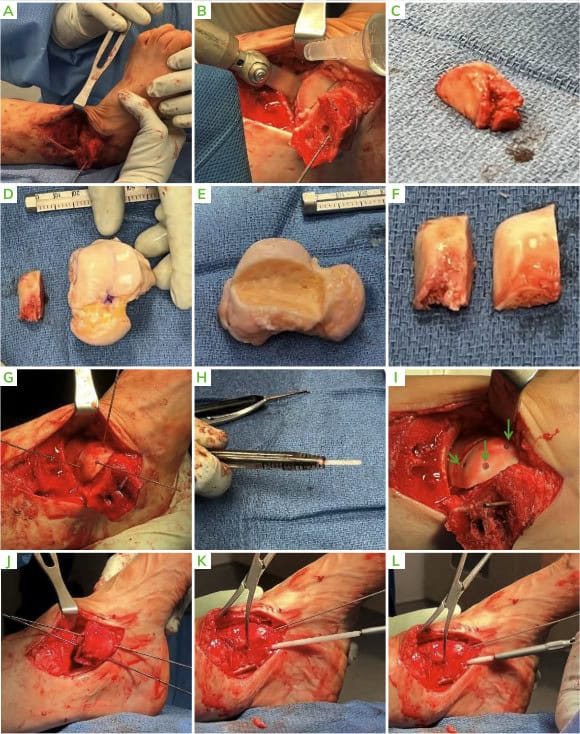
- X-rays demonstrated medial talar shoulder radiolucency suggesting an osteochondral lesion of the talus and distal tibial cyst medially.
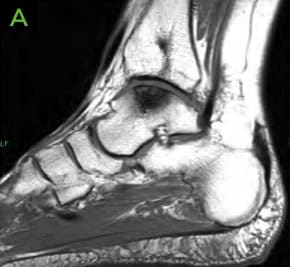
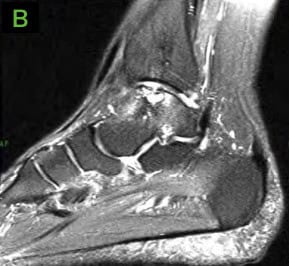
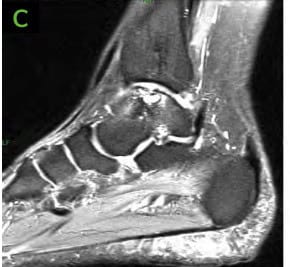
- MRI demonstrated a small curvilinear hypo-intensity in distal tibial metaphysis and epiphysis consistent with cystic changes Medial talar dome shoulder lesion with cystic changes and bone marrow edema. This lesion measured 1.9cm in posterior-anterior (PA) direction and 1.3cm in transverse direction.
- CT scans demonstrated sclerotic regions involving the distal tibia as well as talar the dome, related to chronic changes of prior insufficiency fracture or osteochondral surgeries of recurrent subchondral cystic/osteochondral change involving medial shoulder talar dome.
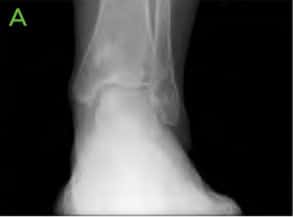
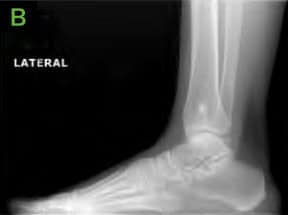
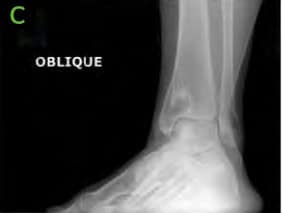
Figure 1: Pre-operative X-rays; [A] AP, [B] Lateral and [C] Oblique. Medial lucency is seen, consistent with tibial cystic changes, as well as medial talar shoulder lucency with underlying sclerosis consistent with dome lesion.
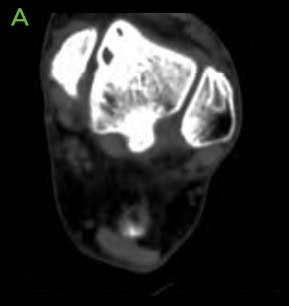
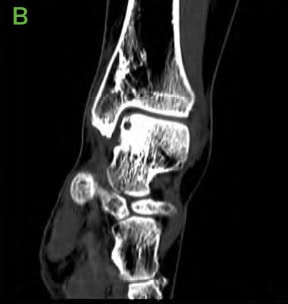
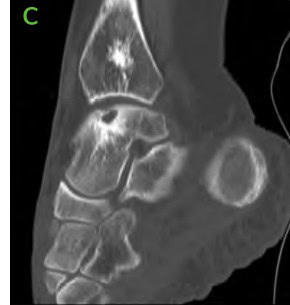
Figure 2: Pre-operative CT scans confirming findings seen on radiographs. [A-C] Medial distal tibial cyst present. Medial talar shoulder dome lesion underlying sclerosis.
WHY OSSIOfiber® IS AN IDEAL CHOICE FOR THIS PATIENT?
Bio-integrative OSSIOfiber® Fixation Nails provide appropriate fixation stability comparable to metal, while safely eliminating within the body over time. This is the optimal choice to avoid permanent hardware retention in the body, hardware incarceration and secondary removal surgeries. In this particular case, there was substantial benefit for non-metal fixation for imaging postoperatively to be able to evaluate integration of the talar allograft. This is specifically useful for evaluation of any early signs of avascular necrosis.
Surgical Procedure
The following OSSIOfiber® fixation implants were used:
- OSSIOfiber® Trimmable Fixation Nail (TFN) 2.4x30mm x3 units
- OSSIOfiber® Cannulated Trimmable Fixation Nail (CTFN) 4.0x70mm x3 units
Accompanying devices/treatment:
- Size matched cadaveric talar Allograft by RTI
- Tactoset® Injectable Bone Substitute by Anika
- Agilon® Moldable Collagen Enhanced Bone Graft by Biogennix
Surgical Technique Steps
The surgeon chose to operate on the patient in two positions.
- Dissection/Access:
A curvilinear incision was made from the proximal medial malleolus distally to level of talar dome. Dissection was carried down to bone and all neurovascular structures were identified and carefully retracted. - Medial Malleolus Takedown Preparation:
Guidewires for 4.0mm OSSIOfiber® CTFN were placed from distal medial malleolus across future osteotomy site into distal tibia under fluoroscopy and were predrilled according to standard technique. Upon exposure of medial malleolus, another wire was placed from medial malleolus obliquely aimed at the medial talar shoulder under fluoroscopy. A sagittal saw was then used to make an osteotomy through medial malleolus to medial talar shoulder along the course of this K-wire. This was completed with an osteotome, and integrity of the deltoid ligament was kept intact. The medial malleolus was then retracted distally for full exposure of the ankle joint. - Talar Site Preparation:
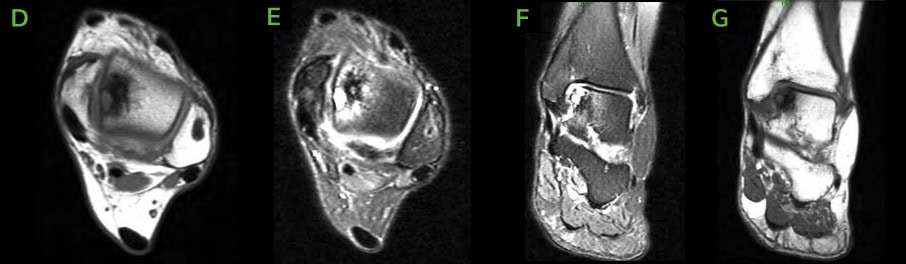
The medial talar dome was noted to have significant cartilaginous defect along the medial shoulder as well as significant softening of subchondral bone. A sagittal saw was used to excise the dorsomedial aspect of talar body. After excision of this segment, significant talar necrosis was noted visually and no bleeding of underlying bone occurred despite no tourniquet use. A 2.0mm drill was then utilized to fenestrate the talus to stimulate bleeding of subchondral bone. - Implant Insertion:
On the back table, a cadaveric implant that had been previously size matched with CT imaging was placed in clamps to prevent cartilaginous damage and was size matched to the resected component and resected using a sagittal saw. The donor allograft was fitted into the previously resected region of the medial talar dome and temporarily fixated with K-wires. A collagen enhanced bone graft was placed between implant and talar body to help facilitate osseous healing. K-wires were over-drilled using standard technique. Three 2.4mm OSSIOfiber® Trimmable Fixation Nails were cut to appropriate measure and tamped in place to fixate the cadaveric graft. The ancillary procedure of the medial tibial; bone cyst was excised with curette and lesion was then packed with cancellous bone, collagen enhanced bone graft and injection of calcium phosphate, which hardened to fill the void. The medial malleolus was reduced into a rectus position and the previously drilled two 4.0mm OSSIOfiber® CTFN measuring 70mm were implanted from the distal medial malleolus to the tibia. Additionally, an anti-glide 4.0mm OSSIOfiber® CTFN was implanted according to standard technique from the medial malleolus segment from medial to lateral across the distal tibia. The deltoid was then stressed under fluoroscopy and noted to not have any insufficiency. Varus stress and anterior drawer test were also completed under fluoroscopy and did not demonstrate any talar tilt or anterior translation of talus. - Closure:
Incisions were flushed with copious amounts of normal sterile saline and closure was achieved using 2-0 Vicryl, 4-0 Monocryl, and 3-0 Nylon.
Patient Post-Operative Protocol Follow-up
The patient was placed into a well-padded posterior splint postoperatively. After 2.5 weeks sutures were removed, and she transitioned to a Controlled Ankle Movement (CAM) boot. At 6 weeks post op the patient stated she fell from scooter onto her operated leg, X-rays showed minor talar graft site gapping without fracture or dislocation.
Patient Follow-up
The patient remained non-weight-bearing for 10 weeks and then transitioned into 50% weight-bearing in boot with crutch assistance for 4 weeks. At 14 weeks she was able to be weight-bearing in boot and began physical therapy.
She began weight-bearing in normal shoes without restriction at 17 weeks. The patient was pain free in her ankle at the 28-week appointment with return to full activity.
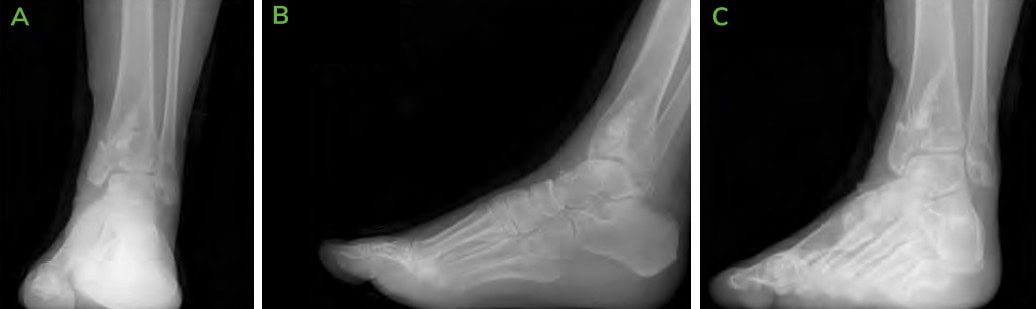
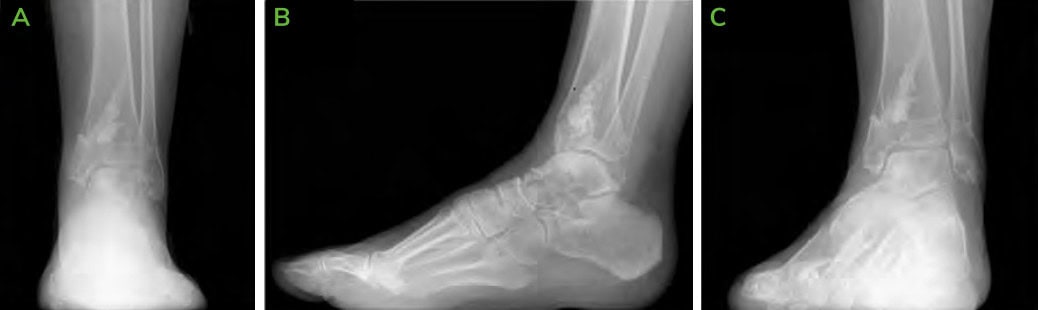
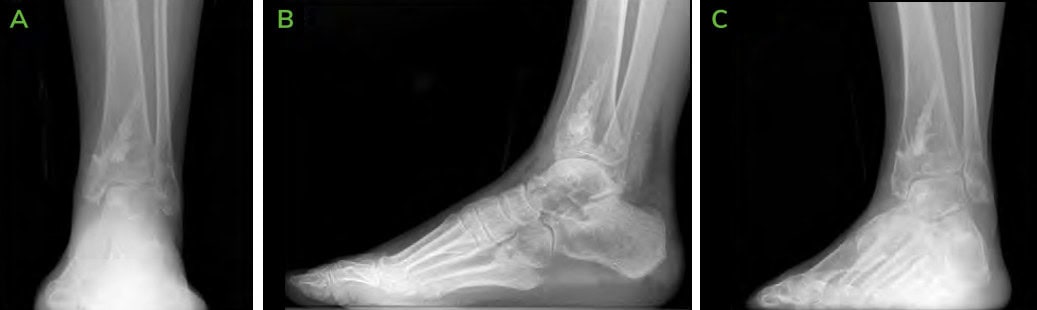
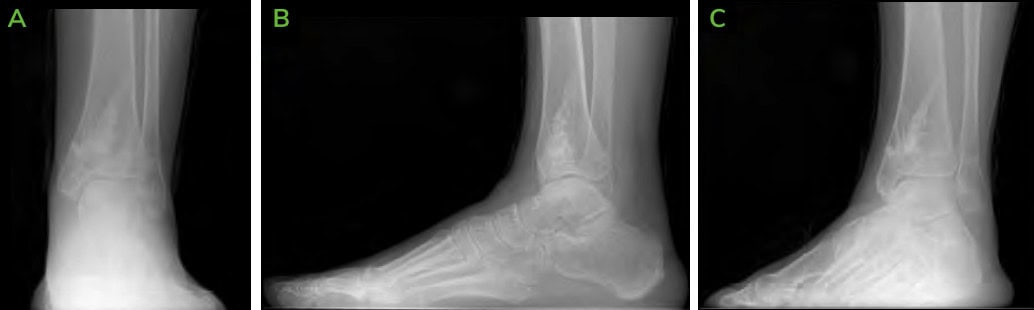
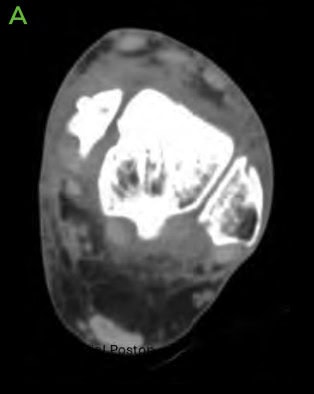
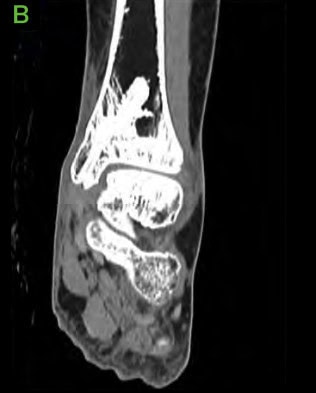
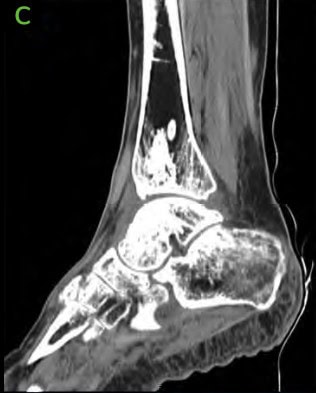
Figure 9: 6.5 months Post-operative CT scans
Summary:
As can be seen from the overview of the treatment options for uncontained shoulder lesions of the talus, treatment options are variable with inconsistency in reported outcomes due to the complexity of these cases. However, it is imperative to offer patients a treatment option that can allow salvage of ankle joint motion with restoration of native anatomy, without necessity for artificial implants. This patient was able to obtain full return to activity without metal implants and salvage restoration of the ankle joint.
References
1. Caracchini, G. et al. Talar fractures: radiological and CT evaluation and classification systems. Acta Bio Medica Atenei Parm. 89, 151–165 (2018).
2. Fortin, P. T. & Balazsy, J. E. Talus fractures: evaluation and treatment. JAAOS-Journal Am. Acad. Orthop. Surg. 9, 114–127 (2001).
3. Buza III, J. & Leucht, P. Fractures of the talus: Current concepts and new developments. Foot Ankle Surg. 24, 282–290 (2018).
4. Kelleher, J., Patel, R., Bua, N. & Vemulapalli, K. Fractures of the talus: where are we now? Orthop. Trauma 37, 17–27 (2023).5, 39–45 (2010).
5. Noori, N., Ouyang, J., Noori, M. & Altabey, W. A review study on total ankle replacement. Appl. Sci. 13, 535 (2022).
6. Zengerink, M., Struijs, P. A. A., Tol, J. L. & van Dijk, C. N. Treatment of osteochondral lesions of the talus: A systematic review.
Knee Surgery, Sport. Traumatol. Arthrosc. 18, 238–246 (2010).
7. Millonig, K. & Gray, T. R. Osteochondral Talar Shoulder Lesion Treatment Options. Clin. Podiatr. Med. Surg. (2024).
8. Bisicchia, S., Rosso, F. & Amendola, A. Osteochondral allograft of the talus. Iowa Orthop. J. 34, 30–37 (2014).
DOC-000 Rev 1.0 01/2025
Medical professionals must use their professional judgement for patient selection and appropriate technique.
Results from case studies are not predictive of results in other cases. Results may vary.
Refer to the product Instructions for Use for warnings, precautions, indications, contraindications and technique.
Some products mentioned in this document may not be currently available or approved for sale in your country.
Speak to your local sales representative/distributor for product availability.
® OSSIO and OSSIOfiber® are registered trademarks of OSSIO Ltd. All rights reserved 2025.
