OSSIOfiber® Trimmable Fixation Nails
All-Natural Healing for Multiple Applications
Bio-Integrative OSSIOfiber® Trimmable Fixation Nail Family
Add Strength and Stability Without Adding Metal
Our unique Trimmable Nail enables all-natural healing and utility in a wide range of surgical applications with the added convenience of sterile, disposable instrumentation.
Bio-Integrative OSSIOfiber® Trimmable Fixation Implant Benefits
Strong, Screw-Like Stability with All the Utility of a Trimmable Nail
OSSIOfiber® Trimmable Fixation Nails are equally strong in the initial stability and fixation strength — and superior in rotational resistance to traditional metal cannulated headless compression screws.*
*Data on File.
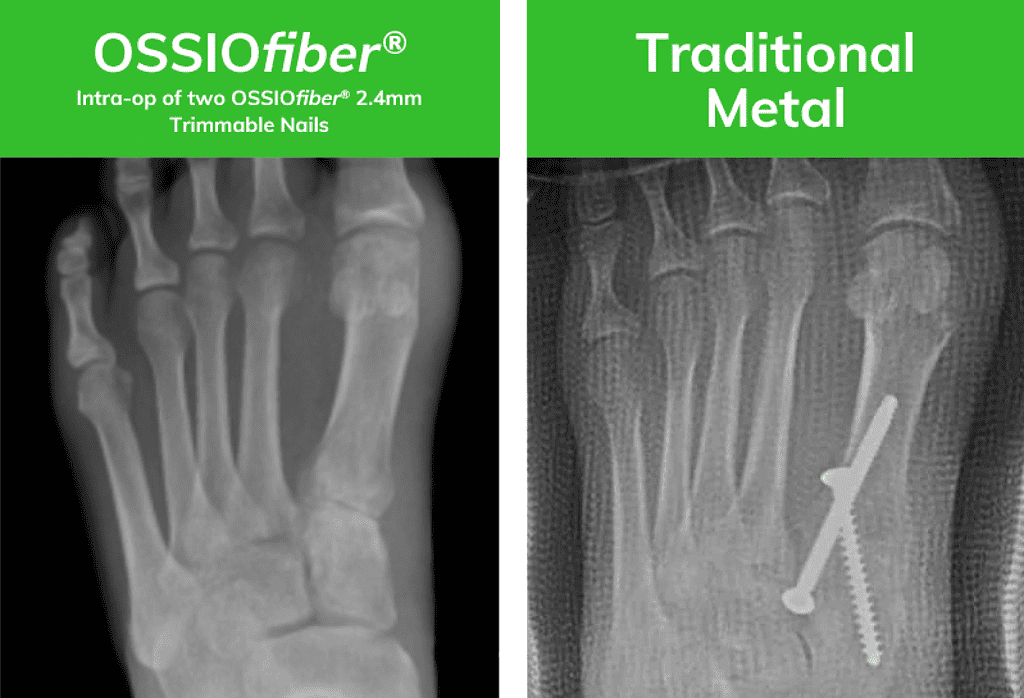
OSSIOfiber® Image courtesy of Dr. Bob Baravarian
X-rays are of two similar patients who underwent an identical surgical approach. One patient was treated with Bio-Integrative OSSIOfiber® while the other was treated with metal compression screws.*
*Results from case studies are not are not predictive of results. Results may vary
Specifications
Applications
Kits
Ordering Info
Bio-Integrative OSSIOfiber® Trimmable Fixation Nails
OSSIOfiber® Cannulated and Solid-Core Fixation Nails allow surgeons to offer patients a strong and bio-integrative solution for a wide range of surgical techniques.
- Comprised of OSSIOfiber® Intelligent Bone Regeneration Technology
- Superior to conventional metal compression screws in compression and pull-out resistance*, and equivalent in shear and flexural stability in an osteotomy construct**. Gradual load sharing minimizes stress risers and promotes return to function
- Up to 5X increased surface area available for bone integration may contribute to a more stable construct***
- Artifact free on CT, X-Ray. MRI safe
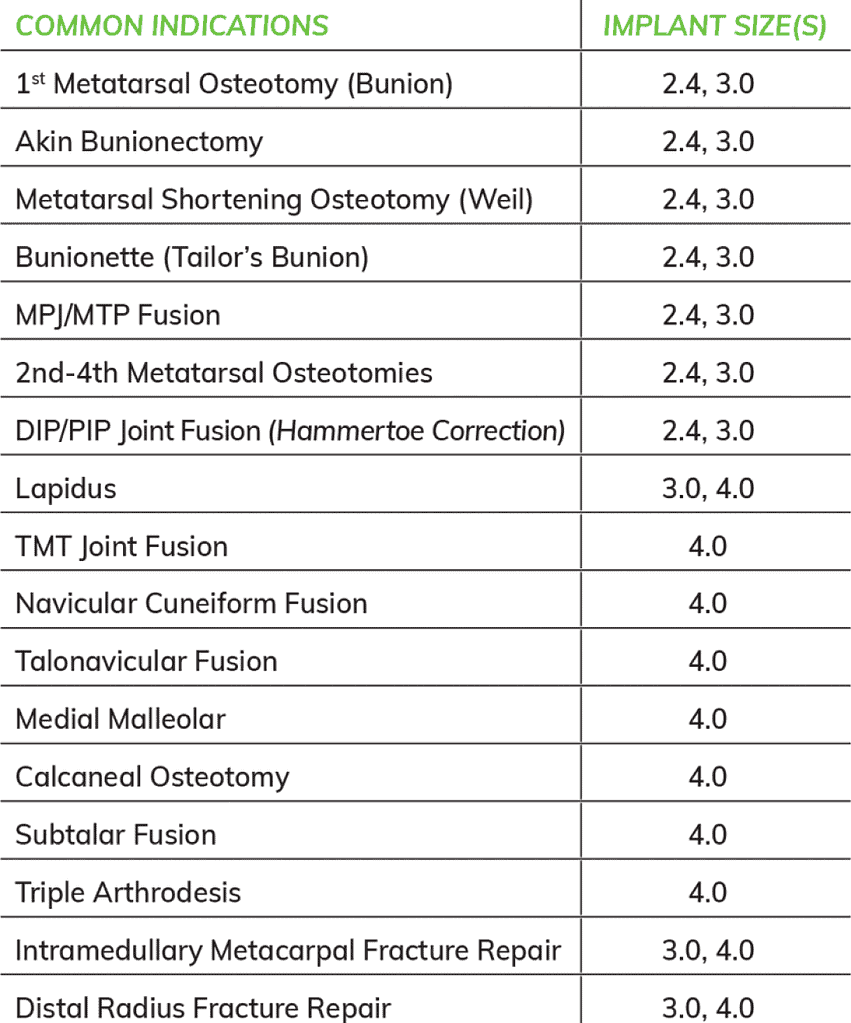
Choose the configuration that best fits your patients’ needs:
Cannulated
- 3.0 X 50mm, One Implant
- 4.0 X 70mm, One Implant
- 4.0 X 100mm, One Implant
Solid-Core
- 2.4 X 30, Two Implants
- 2.4 X 50mm, One Implant
- 4.0 X 50mm, One Implant
- 4.0 X 50mm, Two Implants
* In Vitro Data on File at OSSIO
** In Vitro Flexural Strength Test in a Simulated Osteotomy Construct. Data on File at OSSIO.
*** In Vitro Initial Pull Out Strength of 2.4x50mm OSSIOfiber® Trimmable Fixation Nails. Data on File at OSSIO.

Want to Order? Hear More?
For Product Inquiries, Customer Service, Ordering Information…
Contact Us At:
The OSSIOfiber® Trimmable Fixation Nail
The First and Only Bio-Integrative Trimmable Nail
The Broad Surgical Applications of OSSIOfiber® Trimmable Fixation Nail are achieved with a straight-forward, 5 Step Fixation Process
- Standard Instrumentation accommodates a broad range of surgical applications and techniques
- Visual and tactile confirmation ensures reproducibility
- Easy implant insertion and reduction for secure fixation

Cannulated Trimmable Fixation Nail
For illustration purposes only
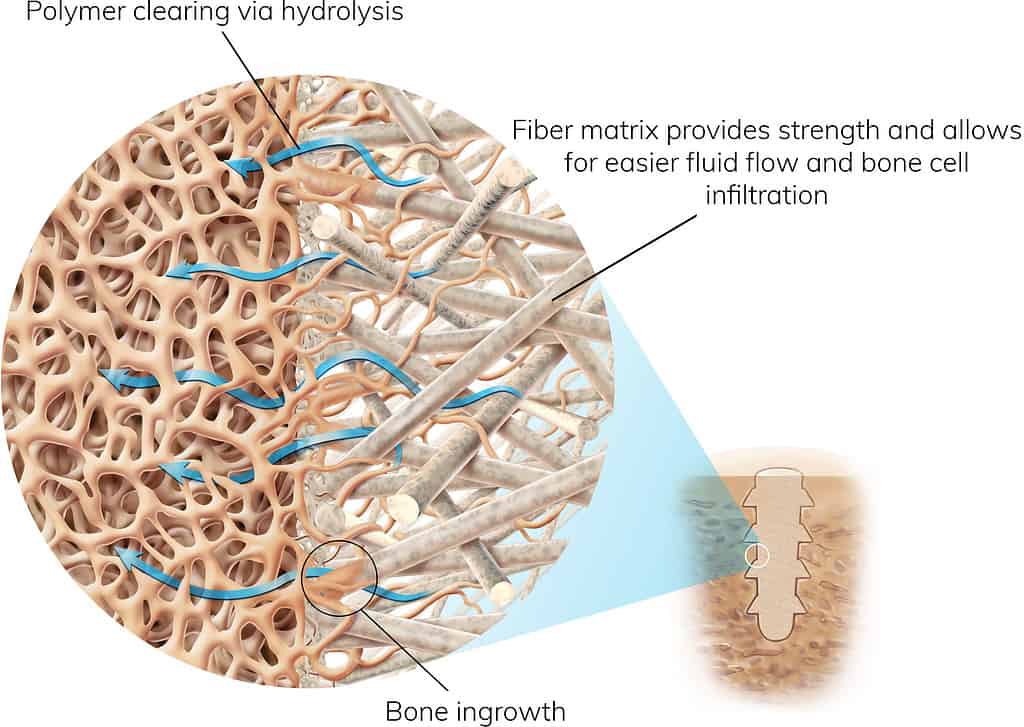
The Science Behind OSSIOfiber® Intelligent Bone Regeneration Technology
A True Breakthrough in Orthopedic Fixation
As a first-of-its-kind implant material, OSSIOfiber® is stronger than cortical bone and leaves nothing permanent behind. It leverages the individual integration mechanism of both material components and internal micro-architecture to achieve the optimal environment for bone healing.
Frequently Asked Questions (FAQ)
What procedures are the OSSIOfiber® Trimmable Nail Systems indicated for?
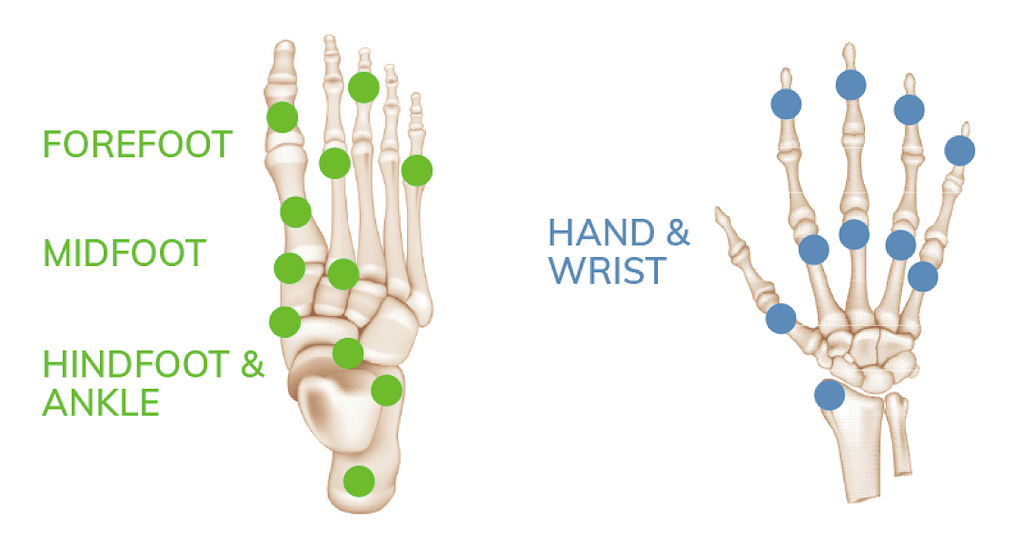
The OSSIOfiber® Trimmable Fixation Nails were designed for broad utility across many surgical procedures including foot/ankle and hand/wrist applications.
The OSSIOfiber® Trimmable Fixation Nail System is indicated for maintenance of alignment and fixation of bone fractures, osteotomies, arthrodesis and bone grafts in the presence of appropriate additional immobilization (e.g. rigid fixation implants, cast, brace). Refer to the product Instructions for Use for indications, contraindications, and additional information.
Refer to the product Instructions for Use for indications, contraindications, and additional information.
Is the OSSIOfiber® Trimmable Fixation Nail System covered under existing reimbursement?
What makes OSSIOfiber® different from a bio-resorbable implant?
OSSIOfiber® is 5x stronger than conventional bio-resorbables and contributes to early bone attachment and subsequent bone integration. Findings from a 2-year preclinical study comparing OSSIOfiber® to conventional bio-resorbables showed that unlike conventional bio-resorbables, OSSIOfiber® integrates in a gradual and predictable way, with no adverse inflammation observed. Please refer to the “Science Behind OSSIOfiber®” page of this website for more detailed information. *Data on file at OSSIO
How long does complete bio-integration take?
The OSSIOfiber® bio-integration process begins shortly after surgery and continues in a gentle, gradual, and predictable way until it is completely incorporated into the surrounding anatomy in roughly 78-104 weeks, as proven in pre-clinical studies. After full bio-integration, the implant is completely gone and replaced by healthy bone. Bone attachment is seen in as little as 2 weeks post surgery, followed by incorporation and ultimate replacement of the implant by tissue – a process that continues until nothing permanent is left behind.
Where is OSSIOfiber® available?
OSSIOfiber® is currently commercially available in the US. If you are interested in using OSSIOfiber®, please fill out the form below and a team member will be in touch.