OSSIOfiber® Hammertoe Fixation System
Same Technique, Breakthrough Material Technology
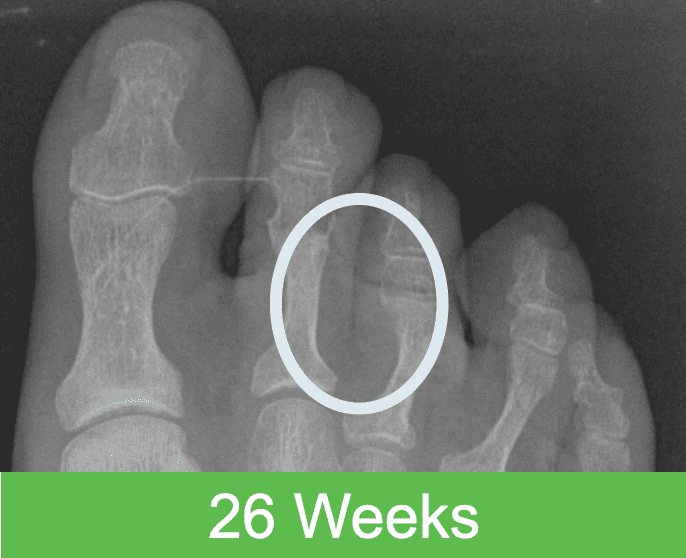
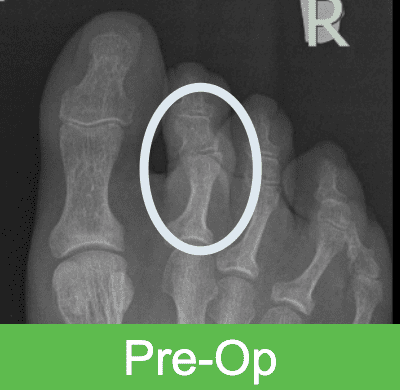
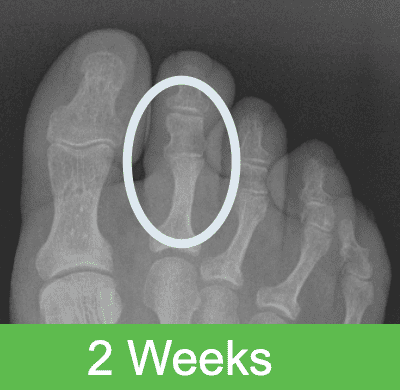
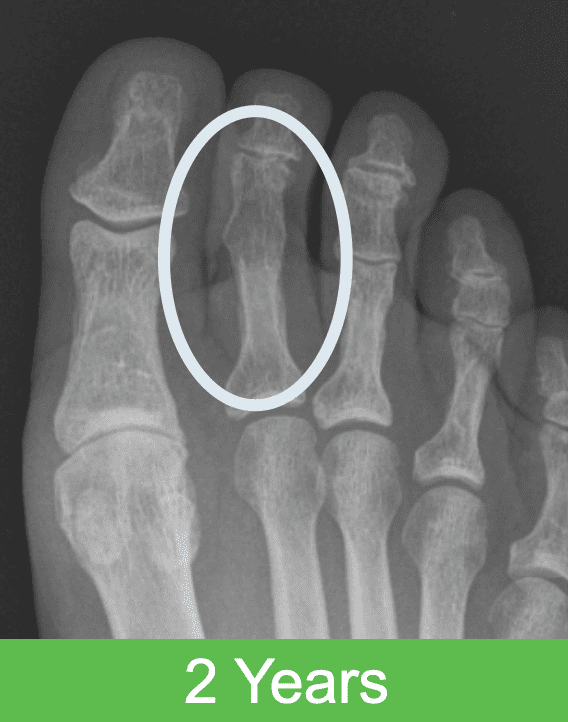
First-in-Human Study: Imaging through 2 Years Post Op
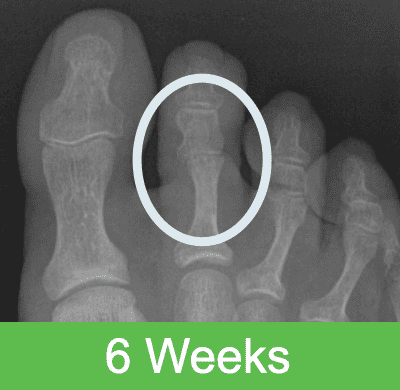
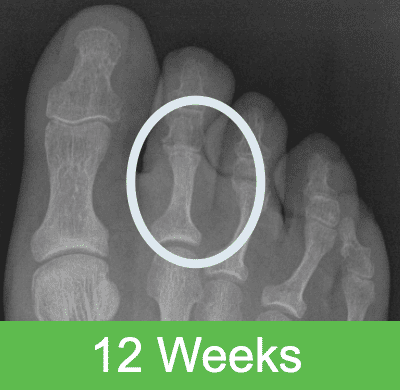
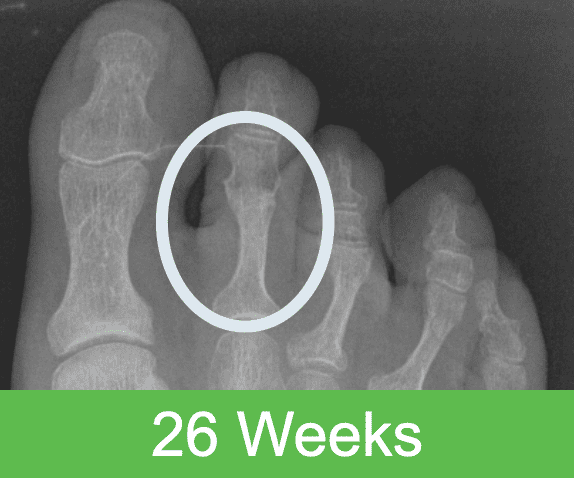
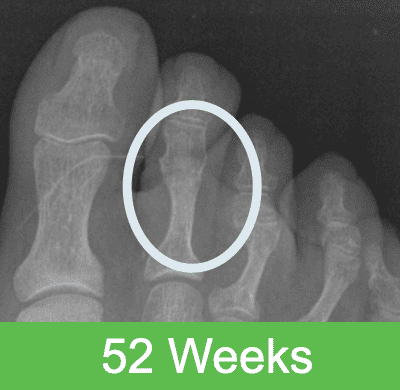
Specifications
Applications
Kits
Ordering Info
OSSIOfiber® Hammertoe Fixation Implant
- Comprised of OSSIOfiber® Intelligent Bone Regeneration Technology
- Strong, secure fixation for PIP fusions
- Ability to fully integrate into surrounding anatomy without adverse inflammation
- Artifact-free on CT and X-ray, MRI safe
Choose the size and orientation that best fits your patients’ needs:
Three Sizes
- 2.5×16 mm (S)
- 2.9×19 mm (M)
- 3.2×21 mm (L)
Two Orientations
- 0º
- 10º

Want to Order? Hear More?
For Product Inquiries, Customer Service, Ordering Information…
Contact Us At:
Surgical Technique Guide for Proximal Interphalangeal (PIP) Fusion
Same Procedure, New Technology
Strong, Bio-Integrative Fixation Begins with a Familiar 5 Step Process
- Instrumentation enables optimal tunnel creation in the middle and proximal phalanx
- Visual and tactile confirmation ensures reproducibility
- Easy implant insertion and reduction for secure fixation
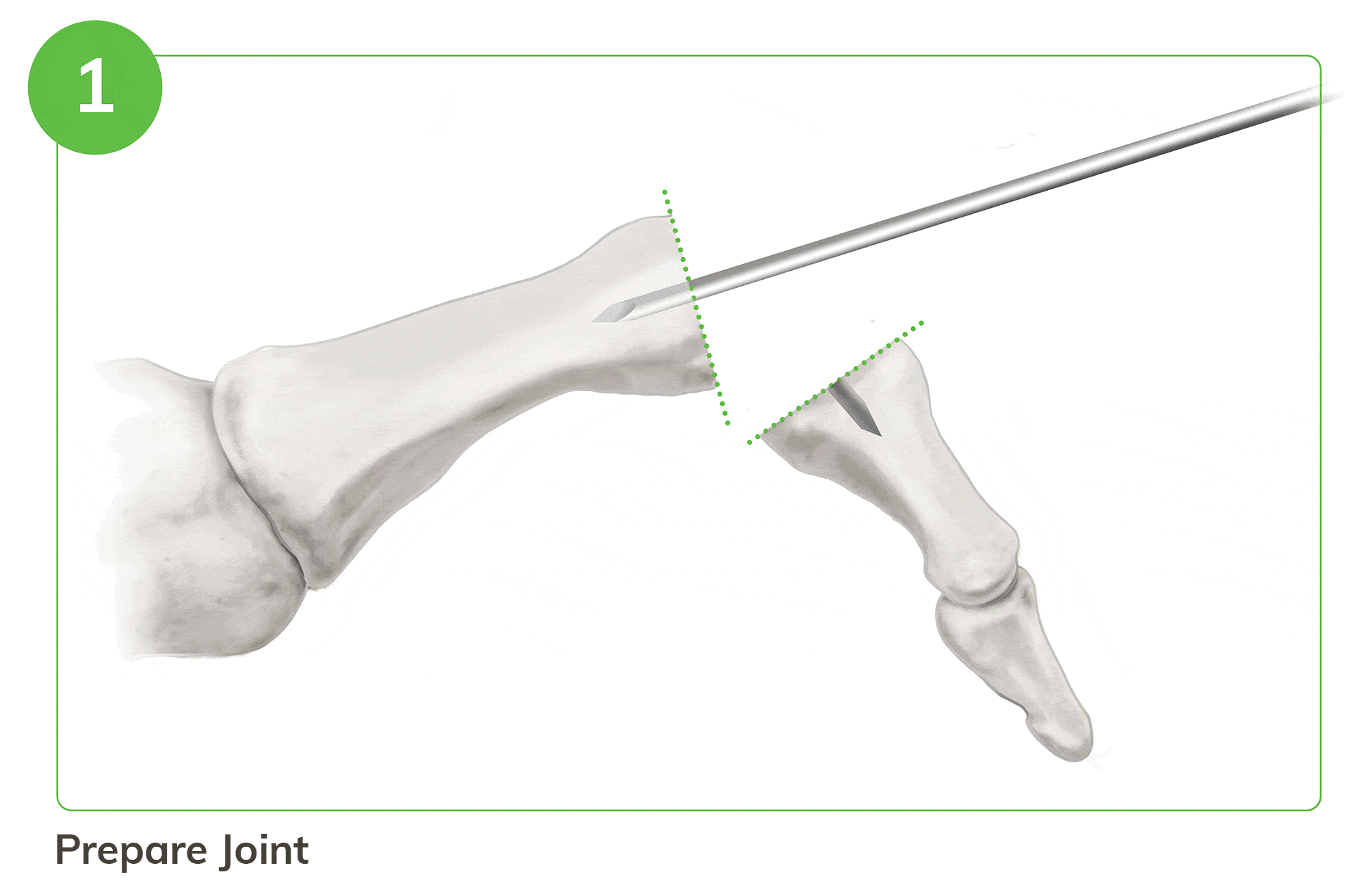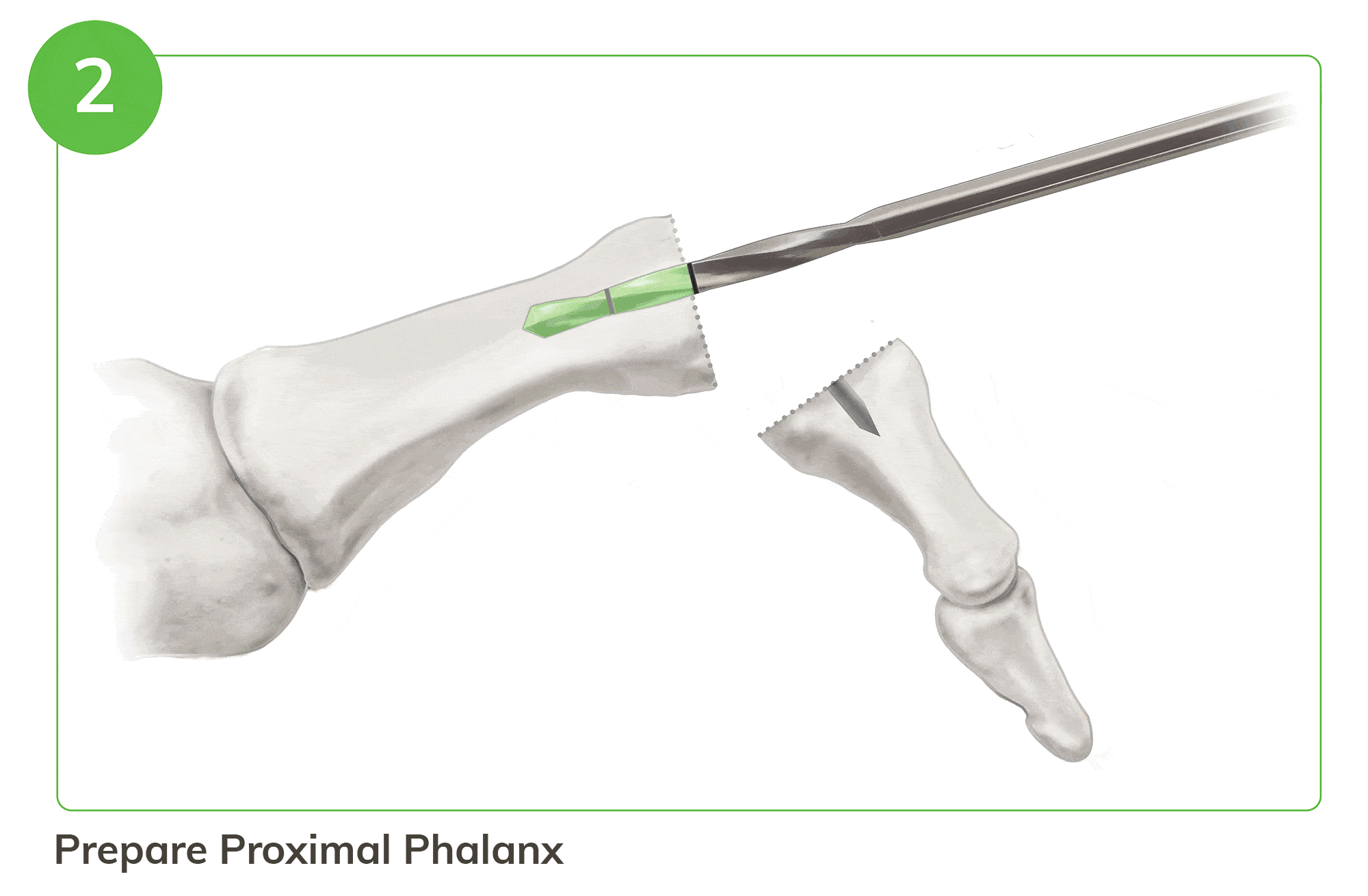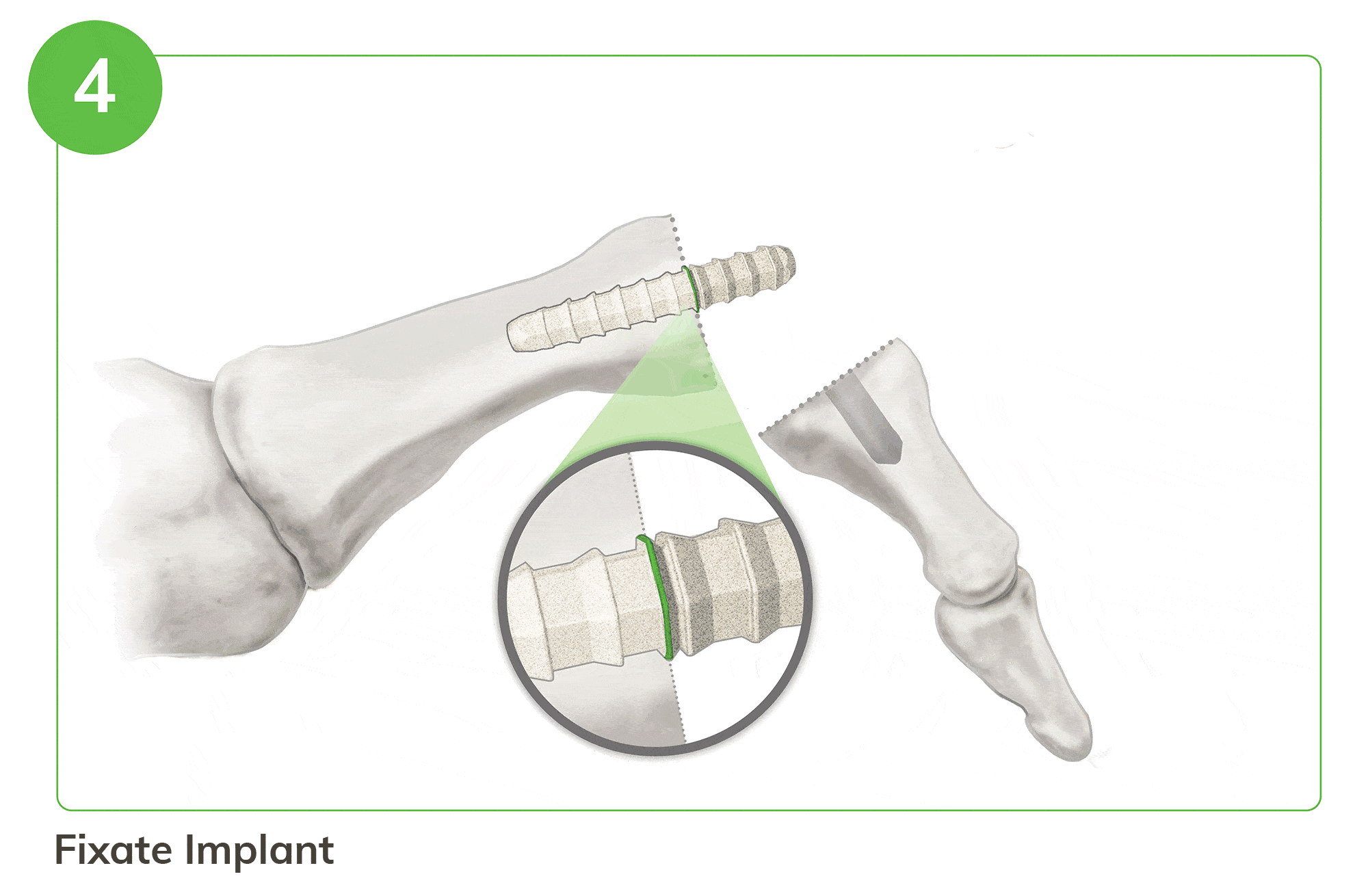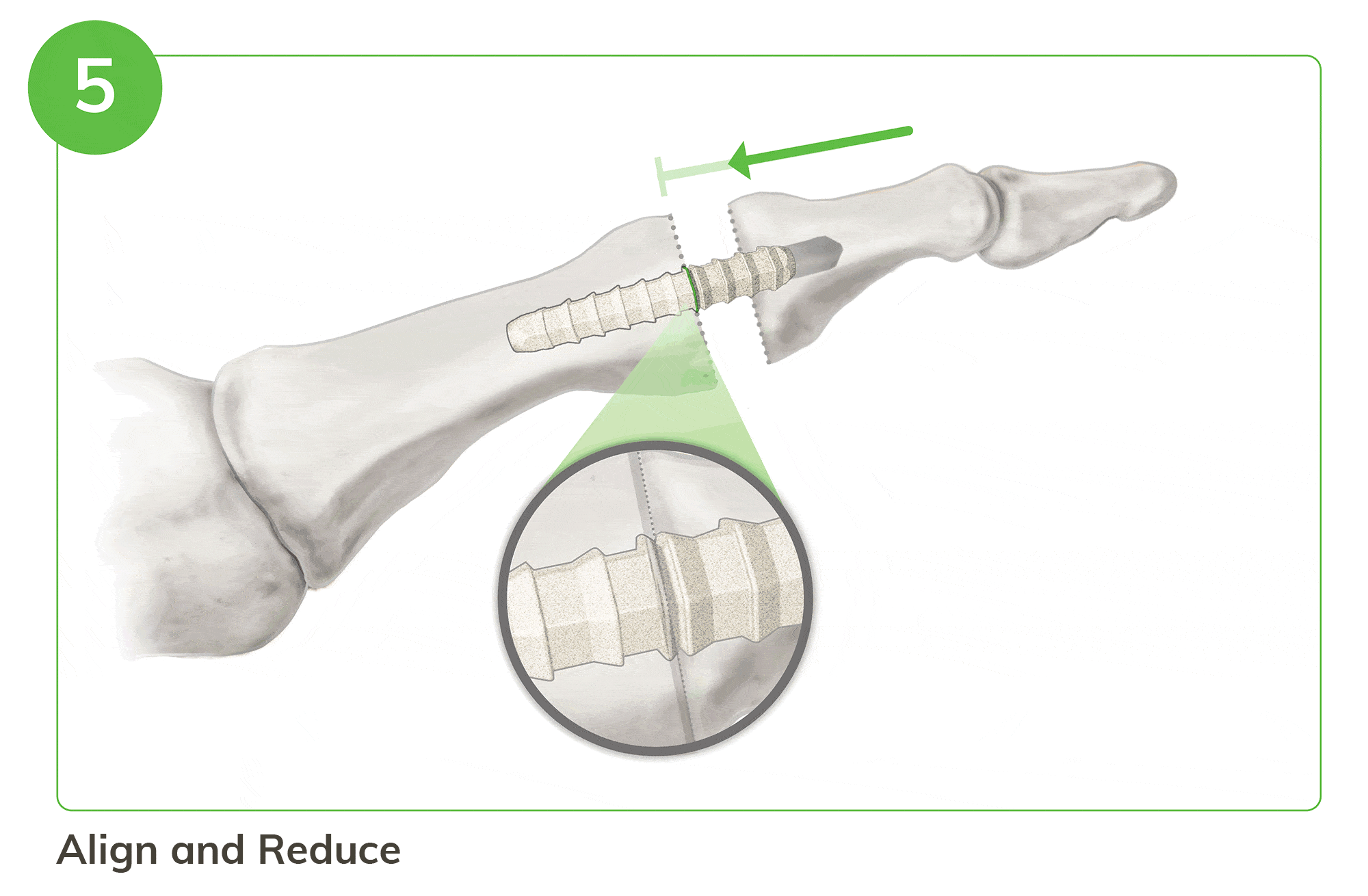
For illustration purposes only
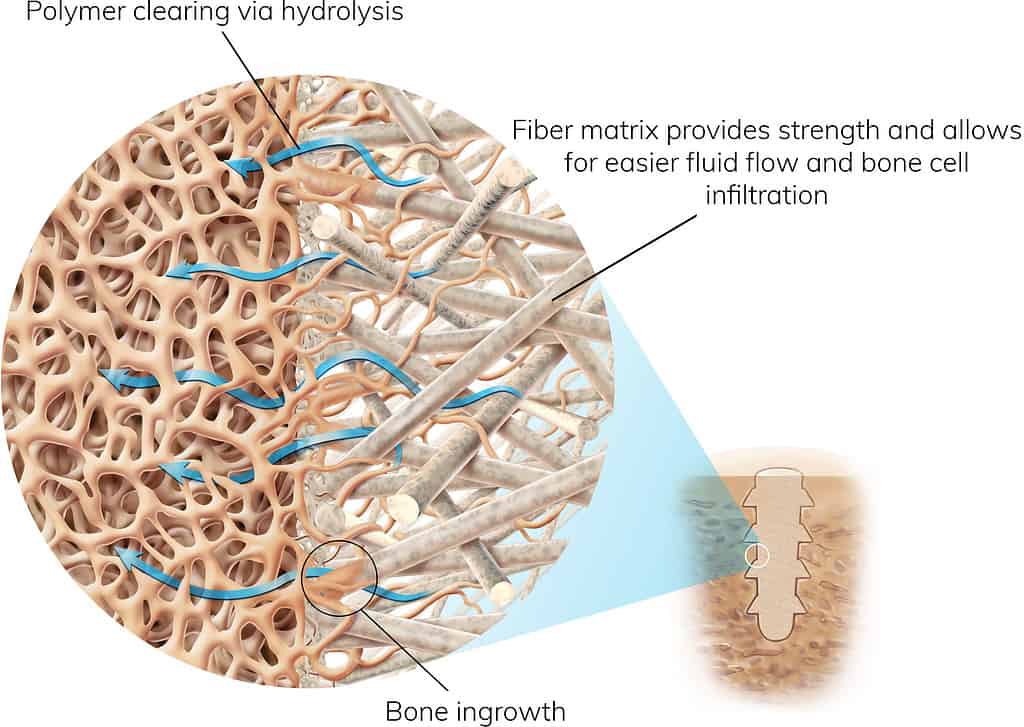
The Science Behind OSSIOfiber® Intelligent Bone Regeneration Technology
A True Breakthrough in Orthopedic Fixation
As a first-of-its-kind implant material, OSSIOfiber® is stronger than cortical bone and leaves nothing permanent behind. It leverages the individual integration mechanism of both material components and internal micro-architecture to achieve the optimal environment for bone healing.
Frequently Asked Questions (FAQ)
Is the OSSIOfiber® Hammertoe Fixation System covered under existing reimbursement?
Yes, it is covered under CPT Code 28285 (Repair of a Hammertoe).
Can OSSIOfiber® be removed, if necessary?
Yes, OSSIOfiber® can be drilled through or cut with instruments such as an oscillating saw, if necessary.
What makes OSSIOfiber® different from a bio-resorbable implant?
OSSIOfiber® is the first and only Bio-Integrative Implant Technology. It is 5x stronger than conventional bio-resorbables and uniquely contributes to early bone attachment and subsequent bone integration. Findings from a 2-year preclinical study comparing OSSIOfiber® to bio-resorbables showed that unlike bio-resorbables, OSSIOfiber® integrates in a gradual and predictable way, with no adverse inflammation observed. Please refer to the “Science Behind OSSIOfiber®” page of this website for more detailed information. *Data on file at OSSIO
How long does complete bio-integration take?
The OSSIOfiber® bio-integration process begins shortly after surgery and continues in a gentle, gradual, and predictable way until it is completely incorporated into the surrounding anatomy in roughly 78-104 weeks, as proven in pre-clinical studies. After full bio-integration, the implant is completely gone and replaced by healthy bone. Bone attachment is seen in as little as 2 weeks post surgery, followed by incorporation and ultimate replacement of the implant by tissue – a process that continues until nothing permanent is left behind.
Where is OSSIOfiber® available?
OSSIOfiber® is currently commercially available in the US. If you are interested in using our products, please fill out the form below and a team member will be in touch.